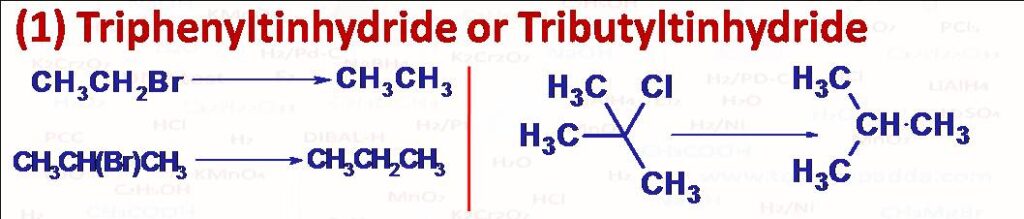
(1) Triphenyltin hydride (C6H5)3SnH and Tributyltin hydride (n-C4H9)3SnH reduce primary, secondary and tertiary alkyl halides to corresponding alkanes. This reaction is carried out by heat, or by irradiation with light or in the presence of a free radical initiator like AIBN (azobisisobutyronitrile). Unlike LiAlH4 here reduction does not take place by SN2 rather takes place by free radical mechanism. Reactivity order for different halides follows as RI>RBr>RCl and for different alkyl it follows as Benzyl > Allyl > tertiary alkyl > secondary alkyl > primary alkyl.
