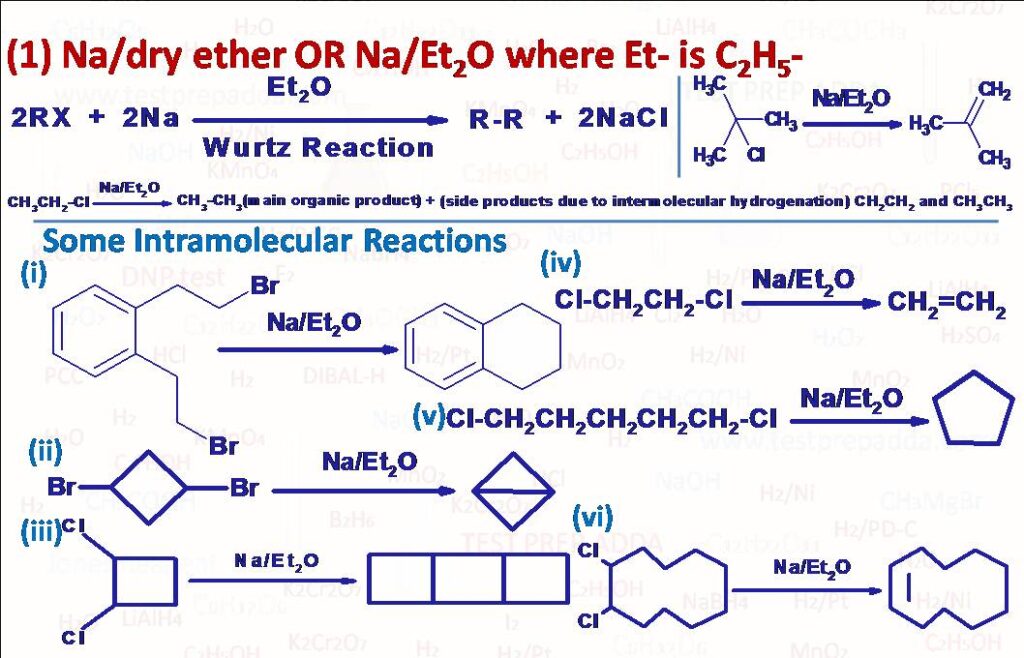
(1) Alkyl halide on treatment with Na in dry ether gives coupling product alkane. This reaction is called Wurtz reaction and generally used for preparation of alkanes with even number of carbon atoms, for alkanes with odd number of carbon atoms the percentage yield is very poor. Generally, Diethyl ether, T.H.F or Dioxane are used as solvent. Methane is not prepared using this Wurtz reaction. Reaction fails to give desired coupling alkane when starting alkyl halide is tertiary and instead gives alkene and corresponding alkane as the major products (due to elimination reaction) and coupling product alkane in negligible amount. Two mechanisms are proposed for this reaction one is ionic mechanism by forming alkyl sodium as intermediate and another free radical mechanism by forming free radical intermediate. Reactivity order is RI>RBr>RCl>RF, and we also get some side reaction products due to intermolecular hydrogenation of intermediate free radical or by elimination method (e.g. when ethyl halide is reacted with Na/Et2O we get three organic products Butane (major), Ethene(minor) and Ethane(minor) and for tertiary butyl chloride we get 2-Methylpropene (major), 2-Methylpropane (major) and 2,2,3,3-Tetramethylbutane(negligible amount)). In intramolecular reaction where same molecule has two halide groups there we can get a ring or double bond in the ring (provided ring size is of 8 or more carbon atoms as per Bredt’s rule). Please note that when we take one compound as aryl halide and another as alkyl halide it is alkylation of aryl halide and known as WURTZ-FITTIG REACTION. Aryl halides (C6H5Cl) also give analogous compounds when treated with sodium in dry ether, in which two aryl groups are joined together (C6H5-C6H5). It is called Fittig Reaction.
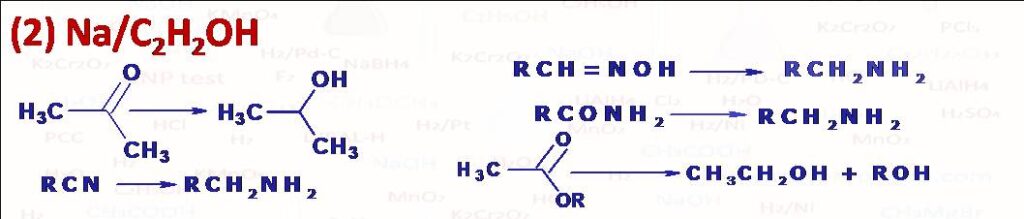
Na and C2H5OH in inert solvent such as toluene or xylene mainly used for the reduction of ester into primary alcohols (Bouveault-Blanc Reduction). Na-C2H5OH can also be used for reduction of aldehydes and ketones into primary alcohols. Alkane nitrile can be reduced into primary amine, oximes and amides are reduced to amines. Alkyl halide (R-X) can be reduced into alkane (R-H). Nonterminal alkyne gets converted in trans alkene.
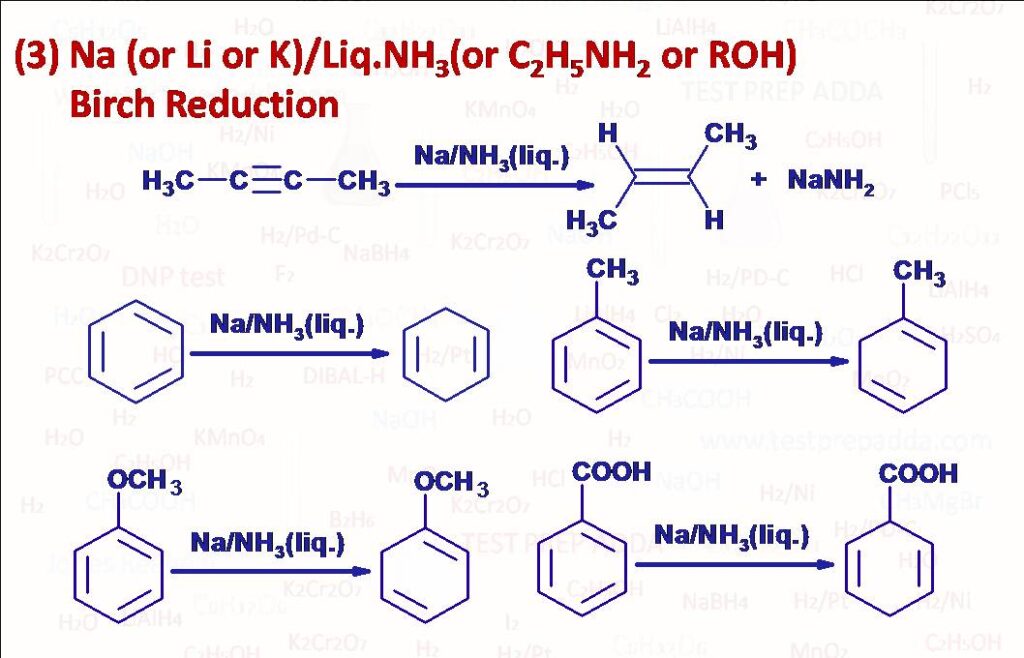
(3) Nonterminal alkynes (internal alkynes) on treatment with Na (or Li or K) in liquid NH3 (or C2H5NH2 or alcohol often ethyl, isopropyl or tert-butylalcohol) give anti-addition of two hydrogen across the triple bond (function is opposite to H2/Lindlar’s catalyst). Benzene ring gets converted into Cyclohexadiene. This is dissolving metal reduction method and known as Birch Reduction. Aromatic rings are also reduced by this reagent and 1,4 addition of two H takes place. When electron releasing groups such as methyl, methoxy etc. are attached they increase the rate and are found on non-reduced positions of the product. When electron withdrawing groups such as -COOH, -CONH2 etc. are attached they decrease the rate and are found on reduced positions of the group. The mechanism involves solvated electrons. Terminal alkyne reacts as an acid and gives sodium alkynide. An ether gets converted into alkane and alcohol.
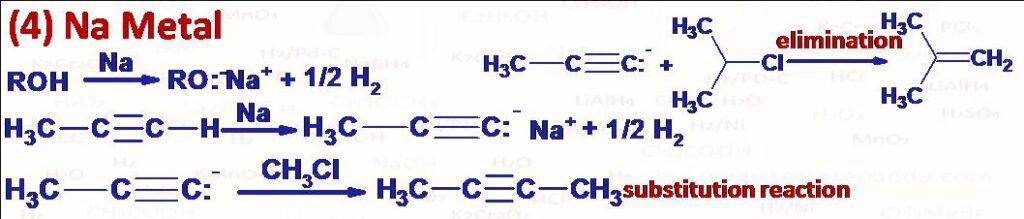
(4) Na (or K) metal reacts with compounds like alcohol, terminal alkyne and liberate H2 gas. Sodium alkynide and alkoxide can act as nucleophile (give substitution product with methyl halide or primary alkyl halide with unbranching at beta carbon) and as base (give elimination product alkene with secondary and tertiary alkyl halide).
