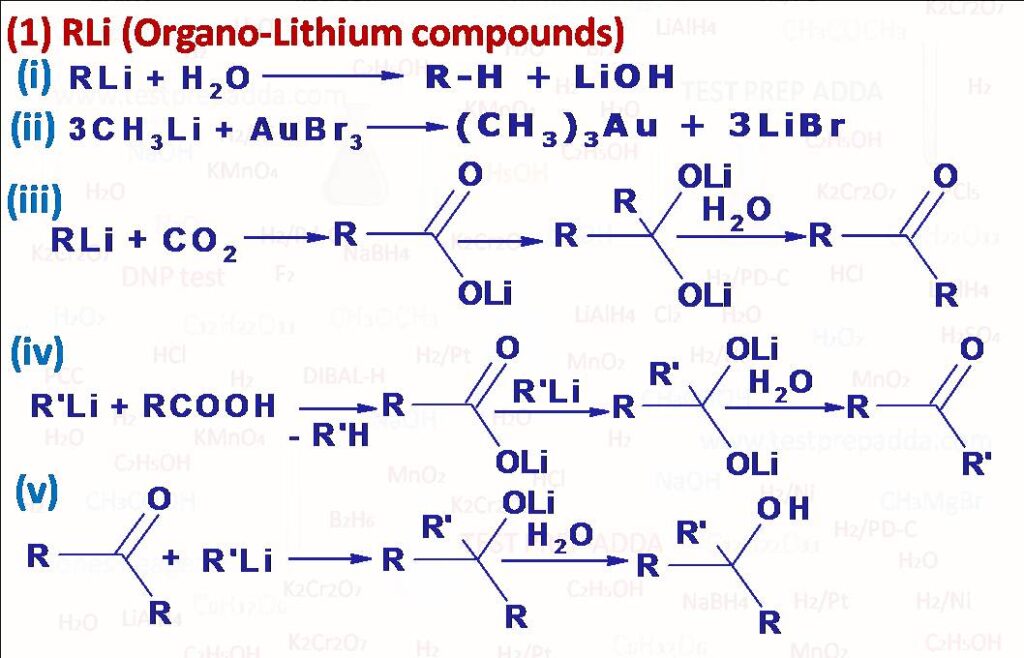
(1) The behavior of organo-lithium (and those of Na and K) is similar to Grignard reagent but Lithium compounds are more reactive. With H2O it gives alkane. With CO2 it gives ketone in a good yield. A ketone is also the main product when a carboxylic acid is treated with organo-lithium compound. It gives alcohols in better yields than Grignard reagent when reacted with aldehydes and ketones. The main difference between organo-lithium compounds and Grignard reagent is organo-lithium can add on to the C=C grouping, when n-butyl-lithium adds on to ethylene under high pressure and after the addition of formaldehyde a mixture of alcohols is obtained. These are step up reactions. Lithium forms various lithium alkenyls with various vinyl halides and this way we can prepare many unsaturated compounds such as alcohols ketones and acids.
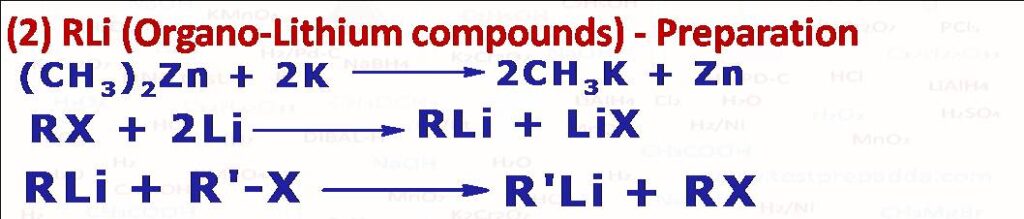
(2) The organo-alkali compounds are generally best prepared by the action of the alkali metal on a dialkyl-mercury or zinc compound. Organo-lithium (RLi) is best prepared by heating an alkyl halide (chloride is preferred as iodide may give Wurtz reaction product) with lithium. Aryl bromides and iodides however give good yields of Li compounds. Those organolithium compounds which cannot be prepared using this method are prepared by exchange reaction).
