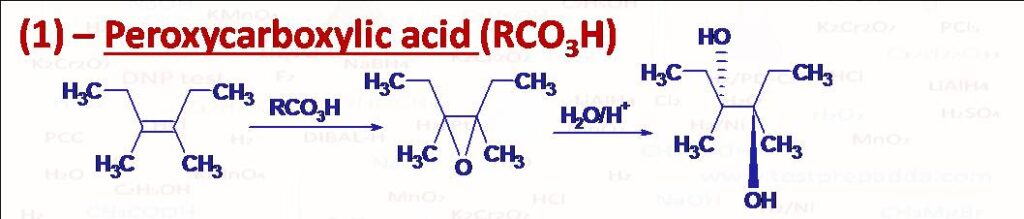
(1) Epoxidation of Alkenes and then hydrolysis: When alkenes are reacted with peroxycarboxylic acids (RCO3H) like Trifluroperoxyaceticacid, Peroxybenzoicacid or Chloroperoxybenzoicacid in solvents such as CHCl3 they form epoxides (Three membered cyclic ethers also called oxiranes). This happens in a concerted manner (syn addition), please note that if there are two double bonds then epoxidation takes place selectively at more substituted double bond. When epoxides are reacted with aqueous acids ring opening occurs to form vicinal glycols. Here nucleophilic attack of water takes place from back side (SN2) so the two -OH groups are attached in an anti-manner (Anti addition), while in cold alkaline KMnO4 and OsO4 two -OH groups get attached from same side.
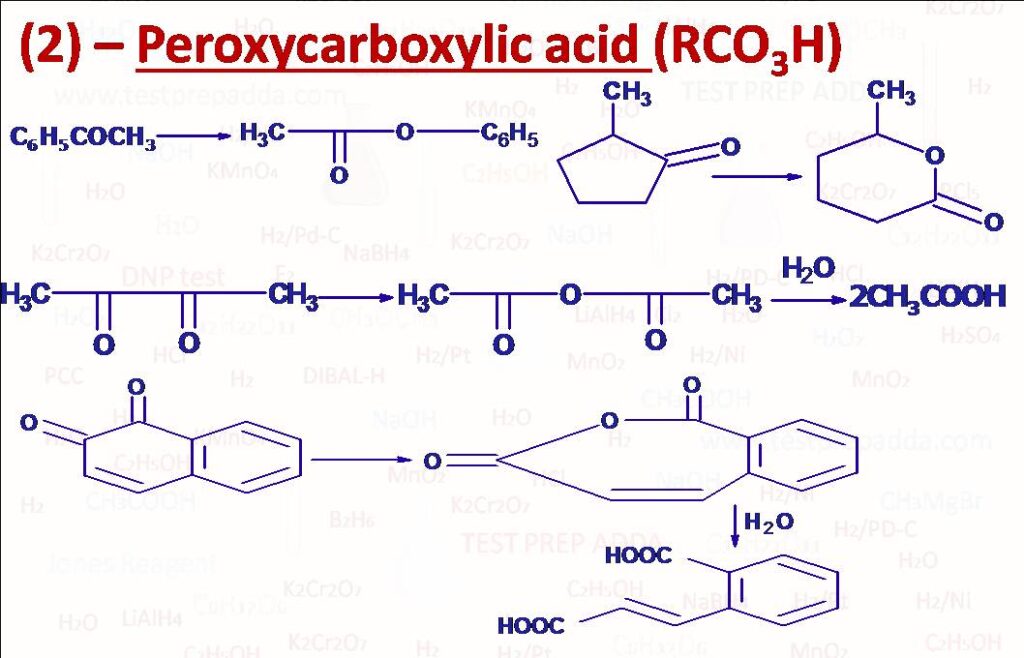
(2) Oxidation of ketones (cyclic ketones) to esters (lactones: these are cyclic esters) (insertion of O between Carbonyl C and generally bulky alkyl group: Baeyer-Villiger rearrangement), please note that during the reaction migration of an alkyl or aryl group takes place and O comes between carbonyl carbon and that group which migrates, the migratory aptitude of the groups for this reaction is in the order tertiary alkyl > secondary alkyl, aryl > primary alkyl > methyl and among aryl it will be p-methoxyphenyl > phenyl > p-chlorophenyl. Oxidation of 1, 2-Diketones and 1, 2-Quinones into acid anhydride and then on hydrolysis into acid. Oxidation of NH2 directly attached to benzene ring into NO2 (mainly CF3CO3H).
