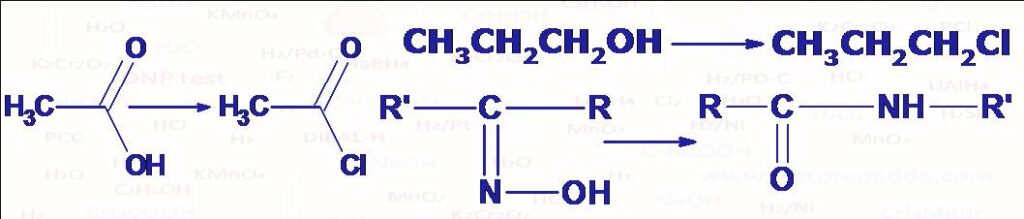
(1) CONVERSION OF ALCOHOL INTO ALKYL CHLORIDE: Alkyl chlorides can be prepared from corresponding alcohols by the reaction with PCl5 (yellow white solid) at room temperature. PBr5 converts alcohols to alkyl bromide. PI5 is highly unstable. The reaction take place by SN2 mechanism (inversion in configuration) where PCl5 gets converted into POCl3 and HCl. Phosphorous Trihalides (PX3) can also be used for the conversion of alcohol into alkyl halide in the same manner (PX3 gets converted into H3PO3), PCl3 and PBr3 are stable but PI3 is unstable so it is generated in situ by heating red P with I2. Please note generally primary and secondary alcohols are converted into alkyl halide, tertiary alcohols mainly undergo elimination so alkyl halides are not obtained in a good yield.
(2) CONVERSION OF CARBOXYLIC ACID INTO ACID CHLORIDE AND PRIMARY AMIDES TO NITRILES: Acid chlorides can be obtained by reacting carboxylic acid with PCl5 or PCl3. Acetic anhydride gives acetyl chloride. Primary acid amides (RCONH2) are converted into RCN by dehydration.
(3) ALDEHYDES AND KETONES are converted into terminal and non-terminal geminal dichlorides respectively.
(4) BECKMANN REARRANGEMENT (CONVERSION OF KETOXIMES INTO AMIDES): Ketoximes (RR\’C=NOH) when treated with acidic reagents such as PCl5, they rearrange to substituted amides (RCONHR’ or R’CONHR). The group that migrates is generally the one anti to -OH of oxime but when attached alkyl groups of oxime are both bulky then mixtures of two amides are possible (RCONHR’ and R’CONHR). The oximes of cyclic ketones give ring enlargement. Among other reagents used are SOCl2, Concentrated H2SO4, HCOOH, Liquid SO2, HMPA, P2O5-Methanesulphonic acid, HCl-HOAc-AC2O, Silica gel and polyphosphoric acid.