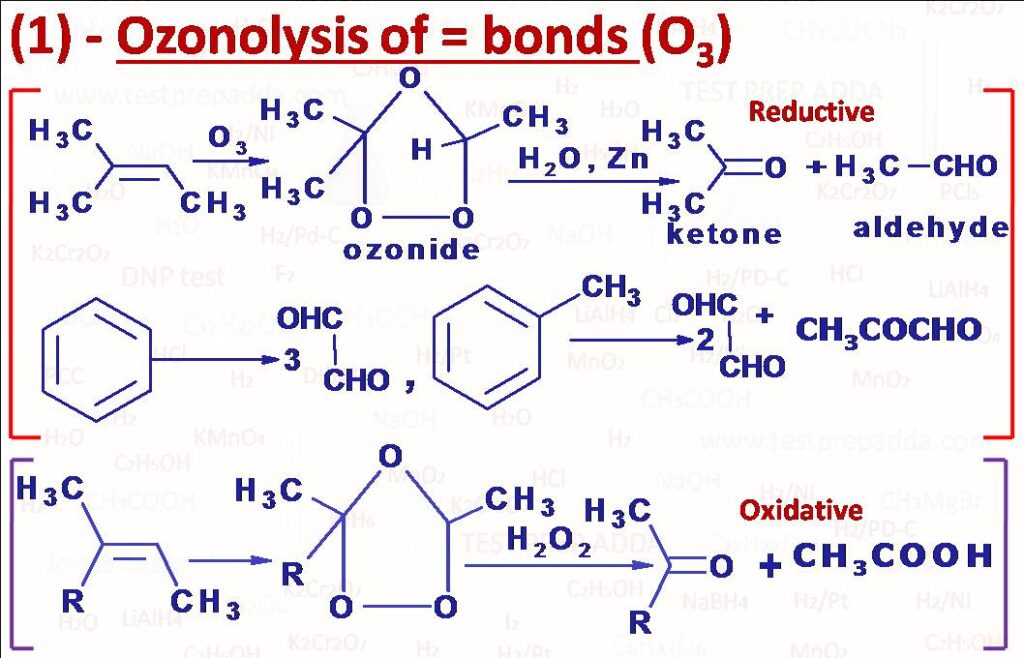
(1) OZONOLYSIS OF DOUBLE BONDS (=) {O3, H2O/Zn}, {O3, H2O2}: There are two parts of the reaction one is reaction with ozone and another is hydrolysis that can be reductive as well as oxidative. Compounds containing double bonds are dissolved in solvents such as CCl4, CHCl3 or CH2Cl2 at low temperature around -78oC, in a concerted cycloaddition unstable molozonide forms which rearranges to give ozonide. REDUCTIVE OZONOLYSIS: Hydrolysis of ozonide is done with H2O and Zn dust or dimethyl sulphide (CH3)2S, we can also use H2/Pd for the reaction. After reaction aldehydes and ketones are obtained depending upon the structure. If double bond carbon bears 1H (or 2H) then it gets converted into aldehyde and no H then ketone. Benzene can be converted into three moles of Glyoxal (CHO-CHO), Toluene can be converted into glyoxal and MeCOCHO in 2:1 molar ratio while o-Xylene can be converted into glyoxal / MeCOCHO / MeCOCOMe in a molar ratio of 3:2:1. OXIDATIVE OZONOLYSIS: When ozonide is reacted with water in the absence of any reducing agent then initially aldehydes and ketones are obtained along with H2O2, aldehyde is further oxidised by H2O2 to carboxylic acid, oxidation of ketone is difficult so ketone remains unaffected as a result after oxidative ozonolysis ketones and carboxylic acids form. This can be effected directly by taking H2O2.
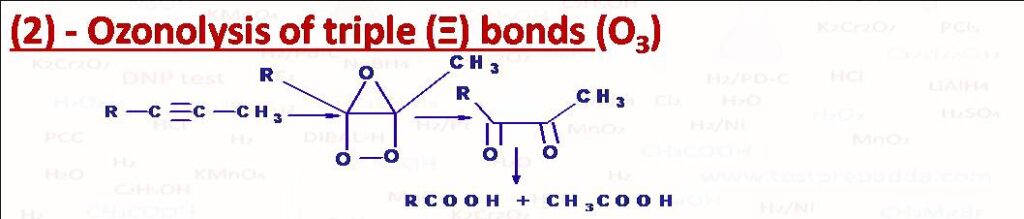
(2) OZONOLYSIS OF TRIPLE BONDS: When ozone is passed through a solution of Alkyne in solvent such as CCl4 or CHCl3, alkyne gets converted into ozonide which on hydrolysis with water first gives alpha dicarbonyl compounds and H2O2. This alpha dicarbonyl compound finally oxidises to carboxylic acids. Each triple bond gives two carboxylic acids.
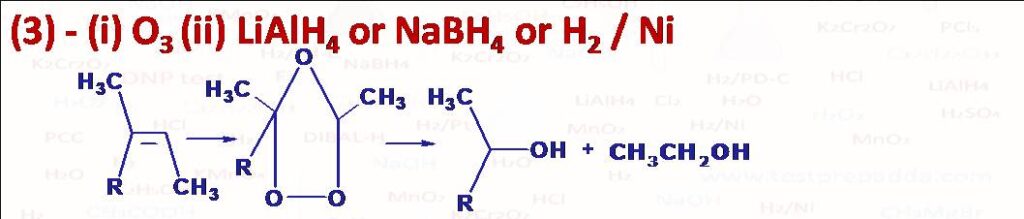
(3) O3, LiAlH4 or NaBH4 or H2 / Ni: This is used to convert double bonds into alcohols.
