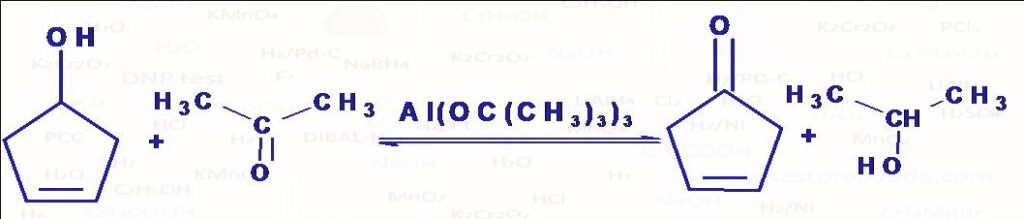
(1) Oppenauer Oxidation: When a ketone in the presence of a base is used as the oxidising agent (it gets reduced to secondary alcohol) the reaction as known as Oppenauer Oxidation. This is reverse of another named reaction Meerwien Ponndorf Verley reduction. The ketones most commonly used are acetone, butanone, cyclohexanone. The most common base is aluminium t-butoxide. The main advantage of this method is its high selectivity. It is used for oxidation of unsaturated sec. alcohol into ketone without affecting unsaturation.
(2) Meerwein Ponndorf Verley Reduction: This reaction is reversible and reverse reaction is known as Oppenauer Oxidation. Generally isopropyl alcohol CH3CH(OH)CH3 (reducing agent: it gets oxidised into acetone CH3COCH3) and aluminium isopropoxide Al(OCHMe2)3 (or aluminium t-butoxide). The reaction is highly specific for aldehydes and ketones. C=C and many other functional groups present remain unaffected.
