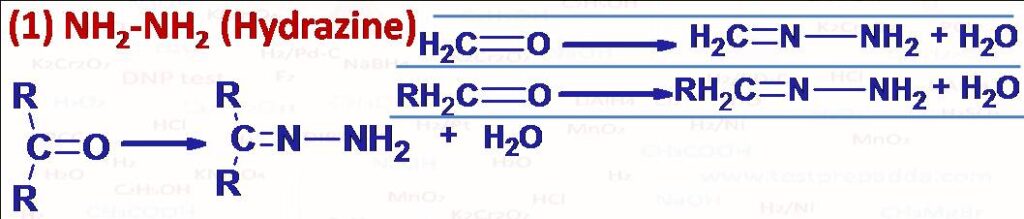
(1) Aldehydes -CHO and Ketones >C=O react with Hydrazine (NH2-NH2) to form Hydrazones (-CH=N-NH2) from Aldehydes and >C=N-NH2 from Ketones). Esters (RCOOR) on reaction with hydrazine give acid hydrazides RCONHNH2 and alcohol ROH.
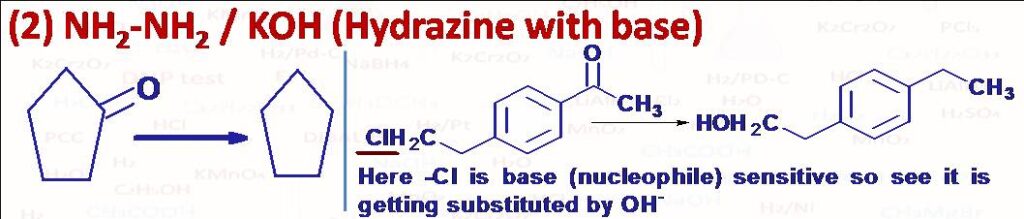
(2) This is a special reagent used to convert aldehydes and ketones (>C=O) into alkane (>CH2). Aldehydes or ketones first react with hydrazine to form the corresponding hydrazone derivatives and then on heating that hydrazone with strong base in a high boiling solvent to give alkane. The reaction is called Wolff-Kishner Reduction. The temperature of the reaction depends upon the strength of base and nature of solvent used. The reduction can be affected at room temperature by using tert-butoxide as base and DMSO (dimethylsulphoxide) as solvent. Please note that this method is avoided to convert >C=O into >CH2 if compound has any base sensitive group like halogen, it can get substituted by base (nucleophile) or elimination may take place of that group. Another reagent which can be used to convert >C=O into >CH2 is Zn-Hg/HCl (Clemmensen Reduction).
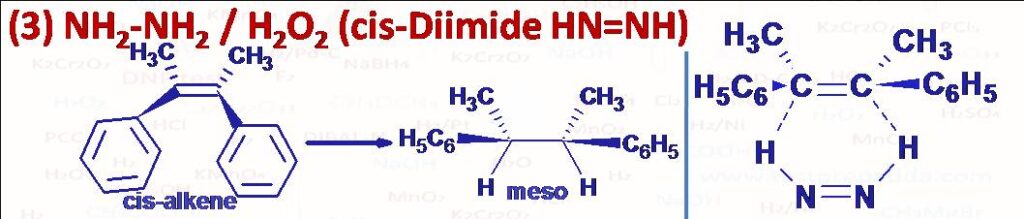
(3) NH2-NH2 gets oxidised with H2O2 to give Diimide (HN=NH) cis and trans. The cis-diimide is used to transfer two hydrogen atoms to alkene to prepare alkane and diimide itself gets converted into N2. This transfer takes place in a concerted manner via a six membered cyclic transition state (a pericyclic reaction). This is a syn addition and stereospecific reaction.
STEREOSPECIFIC REACTION: A given stereoisomer reactant leads to one product while another stereoisomer leads to a different opposite product from stereochemistry point of view.
STEREOSELECTIVE: Any reaction in which only one of a set of stereoisomers is formed exclusively or predominantly is stereoselective. Please note that all stereospecific reactions are necessarily stereoselective, but the converse is not true. In this hydrogen transfer reaction cis alkene gives meso product while trans alkene gives enantiomers (d,l) or we can say racemic mixture. Another reagent which can be used for similar hydrogen transfer to akene in the same manner is cyclohexene in presence palladium catalyst, where cyclohexene itself gets converted into benzene.
