(1) Sabatier-Senderens Reaction
Catalytic hydrogenation H2/Ni. Please refer H2/Catalyst.
(2) Wurtz Reaction
Please refer Reagent Na for Wurtz reaction, Fittig reaction and Wurtz-Fittig reaction.
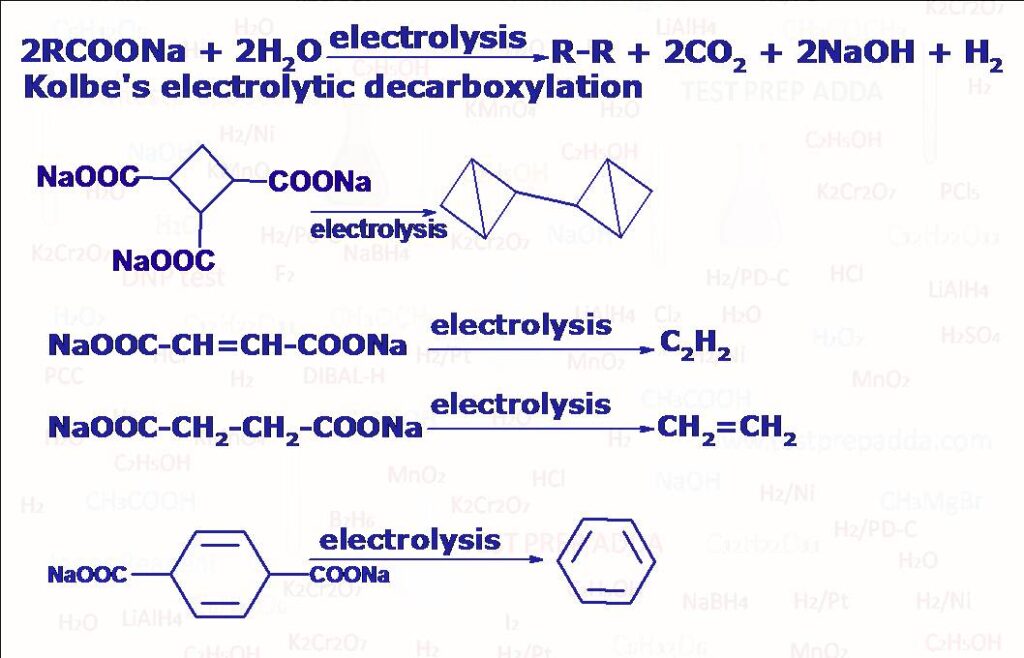
(3) Kolbe’s Electrolytic Reduction
It is a method for getting alkane using decarboxylation is ELECTROLYTIC DECARBOXYLATION also known as Kolbe’s electrolytic reduction. When sodium or potassium salt of carboxylic acid (2RCOONa) is electrolysed in water, DMF or methanol using platinum electrodes we get an alkane R-R. There is another decarboxylation method called SODALIME DECARBOXYLATION to convert sodium or potassium salt of carboxylic acid to corresponding alkane. Please refer Reagent NaOH.
(4) Kolbe-Schmidt Reaction
Please refer Reagent NaOH.
(5) Frankland Readuction
Please refer Zn.
(6) Corey House Synthesis
Please refer Gilman reagent R2CuLi.
(7) Clemmensen Reduction
Please refer reagent Zn-Hg/Cl.
(8) Wolff Kishner Reduction
Please refer reagent NH2-NH2.
(9)Mozingo Reduction
This is a method to prepare alkane from aldehydes and ketones. First aldehydes or ketones (R2C=O) are reacted with Ethane-1,2-dithiol (HS-CH2-CH2-SH) in the presence of any Lewis acid such as BF3 to give cyclic dithioketal which on reduction with H2/Ni gives alkane (RCH2R).
(10) Hoffmann Elimination
Hoffmann Elimination also known as exhaustive methylation to produce alkene. First an amine is methylated with excess of CH3I to produce a quaternary ammonium salt which is when heated with silver oxide and water gives an alkene (if quaternary ammonium salt has beta H) and a tertiary amine. Less substituted alkene forms as major product (regioselective). The elimination is anti-elimination.
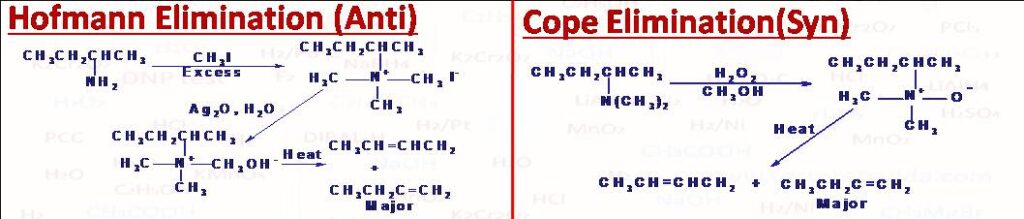
(11) Cope Elimination
When a tertiary amine is treated with H2O2/C2H5OH (or RCO3H or Caro’s acid H2SO5) a tertiary amine oxide is formed which when heated (if possess beta H) at around 150oC produces an alkene and N,N-Dialkylhydroxylamine. The reaction is stereoselective syn elimination and takes place in a concerted manner via five membered cyclic transition state. Less substituted alkene generally forms as major product. Another method where alkene with syn elimination is obtained pyrolysis of acetate ester. Using this method an alcohol can be converted into alkene. first alcohol is converted into its acetate ester by treating it with acetic anhydride then on heating at around 300 to 600oC pyrolysis of acetate ester takes place (six membered cyclic transition state) to give acetic acid and alkene.
(12) Birch Reduction
Please refer reagent Na.
(13) Hydroboration Oxidation
Please refer reagent NH2-NH2.
(14) Oxymercuration Demercuration
Alkenes on reaction with mercuric acetate in aqueous solution (oxymercuration) then reduction with NaBH4 (Demercuration)yield alcohols. Overall, it is addition of water with Markovnikov’s rule and no rearrangement. If we take ROH in place of H2O then ethers are formed instead of alcohols and reaction is called Solvomercuration Demercuration.
(15) Haloform Reaction
Please refer reagent NaOH.
(16) Aldol Condensation
Please refer reagent NaOH.
(17) Cannizzaro Reaction
Please refer reagent NaOH.
(18) Perkin Reaction
Aromatic aldehydes undergo condensation with aliphatic acid anhydride having at least two alpha hydrogen atoms when heated with sodium salt of acid to give beta-aryl-alpha-beta-unsaturated aldehydes. The reaction is called Perkin Reaction.
(19) Knoevenagel Reaction
Active hydrogen compounds (e.g. CH3COCH2COOC2H5) condense with aldehydes and ketones known as Knoevenagel condensations. These aldol like condensation are catalysed by weak bases such as amines {(C2H5)2NH}.
(20) Tishchenko Reaction
All aldehydes (with or without alpha H atoms) can be made to undergo self-oxidation and self-reduction like Cannizzaro reaction by treatment with aluminium ethoxide (C2H5O)3Al but instead of carboxylic acid and alcohol ester is formed. The reaction is known as Tishchenko reaction.
(21) Reformatsky Reaction
Aldehydes and ketones react with esters of alpha bromocarboxylic acids in presence of metallic Zn in anhydrous ether to form beta-Hydroxy esters. The reaction is known as Reformatsky Reaction. These esters can be dehydrated to give alpha-beta- unsaturated esters.
(22) Benzoin Condensation
When benzaldehyde (or other aromatic aldehyde) (C6H5CHO) is heated with aqueous ethanolic KCN it dimerises to give alpha hydroxyketone known as benzoin (C6H5COCHOHC6H5). Benzoin on oxidation with dilute nitric acid gives Benzil (C6H5COCOC6H5). When Benzil is heated with aqueous NaOH it undergoes rearrangement to form Benzilic acid . The reaction is known as benzilic acid rearrangement. Aliphatic 1,2-diketones also undergo similar rearrangement.
(23) Pinacol Pinacolone Rearrangement
Please refer reagent H2SO4.
(24) Baeyer Villiger Rearrangement
Please refer reagent per-oxyacid RCO3H.
(25) Rosenmund Reduction
Acid halides are reduced to aldehydes when treated with H2 gas in boiling Xylene in the presence of Lindlar’s catalyst. Please refer H2/Catalyst.
(26) Beckmann Rearrangement (CONVERSION OF KETOXIMES INTO AMIDES)
Ketoximes (RR’C=NOH) when treated with acidic reagents such as Concentrated H2SO4, they rearrange to substituted amides (RCONHR’ or R’CONHR). The group that migrates is generally the one anti to -OH of oxime but when attached alkyl groups of oxime are both bulky then mixtures of two amides are possible (RCONHR’ and R’CONHR). The oximes of cyclic ketones give ring enlargement. Among other reagents used are SOCl2, PCl5, HCOOH, Liquid SO2, HMPA, P2O5-Methanesulphonic acid, HCl-HOAc-AC2O, Silica gel and polyphosphoric acid. Please refer reagent H2SO4.
(27) Hofmann Bromamide Reaction
Please refer reagent NaOH.
(28) Reimer Tiemann Reaction
Please refer reagent CHCl3.
(29) Gattermann Reaction
Arenediazonium chlorides and bromides when heated with Cu powder in presence of corresponding hydrogen halide acid give aryl chlorides and bromides respectively. Reactions are called Gattermann reactions. This is a modification of Sandmeyer Reaction.
(30) Gattermann Koch Reaction
When benzene or its derivative is treated with carbon monooxide (CO) and hydrogen chloride (HCl) in the presence of anhydrous aluminium chloride (AlCl3) or cuprous chloride (CuCl). It gives benzaldehyde or substituted benzaldehyde. The reaction is known as Gattermann-Koch reaction.
(31) Sandmeyer Reaction
When Arenediazonium chlorides and bromides when heated with Cuprous chloride in presence of HCl and Cuprous bromide in presence of HBr, the corresponding aryl chlorides and bromides are formed respectively. Reactions are called Sandmeyer reactions.
(32) Finkelstein Reaction
Alkyl iodides R-I are often prepared by the reaction of alkyl chlorides (R-Cl) or alkyl bromides (R-Br) with NaI in dry acetone. This reaction is known as Finkelstein Reaction. This is an example of halogen exchange. NaCl (or NaBr) formed during the reaction is precipitated in dry acetone and facilitates the forward reaction according to Le Chatelier’s Principle. The reaction proceeds by SN2 reaction.
(33) Swarts Reaction
The best method to synthesis alkyl fluorides is heating an alkyl chloride or bromide in presence of metallic fluoride such as AgF, Hg2F2, SbF3 or CoF2. The reaction is known as Swarts Reaction. This is another example of halogen exchange reaction.
(34) Friedel Crafts Alkylation and Acylation Reactions
When benzene or substituted benzene is treated with alkyl chloride (R-Cl), alky bezene forms and reaction is known as Friedel Craft Alkylation. Similarly, if benzene or substituted benzene is treated with acid chloride (RCOCl) or acid anhydride (carboxylic acid with H2SO4), acylbenzene forms (C6H5COCl) an aromatic ketone. The reaction is known as Friedel craft acylation. Fridel Crafts reactions are not given by benzene rings connected with meta directors (deactivated rings). Aniline does not give Friedel Crafts rection as N of aniline forms a complex with Lewis acid used in reaction. The limitation associated with Friedel Crafts acyalation is if alkyl group of acid chloride is tertiary then acylenium ion breaks into tertiary carbocation and CO and this way tertiary alkyl benzene form which undergoes further acylation (para position).
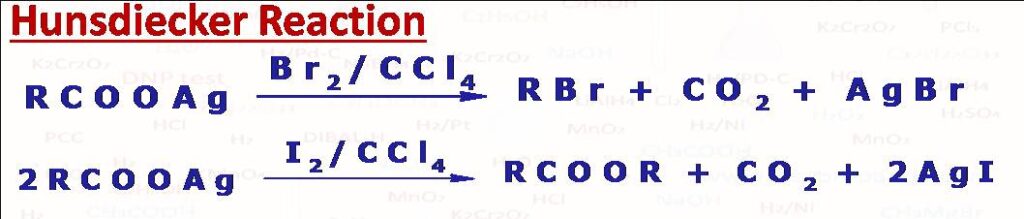
(35) Hunsdiecker Reaction
This reaction is decarboxylative bromination of silver carboxylates. When silver salt of carboxylic acid is heated with Br2 in dry CCl4 an alkyl bromide is formed with evolution of CO2. The reaction is step down reaction where product possess one carbon less than the starting substrate. The reaction follows free radical mechanism where first acyloxy then alkyl free radical is formed. We can take Carboxylic acid and HgO in place of silver carboxylate to get the same product. Please note When iodine is the reagent, a 1:1 ratio (RCOOAg:I2) gives alkyl iodide but if the ratio is 2:1 (RCOOAg:I2) then ester(RCOOR) is formed instead of alkyl iodide. The reaction is called Simonini reaction. The same product is obtained with carboxylic salt of Pb.
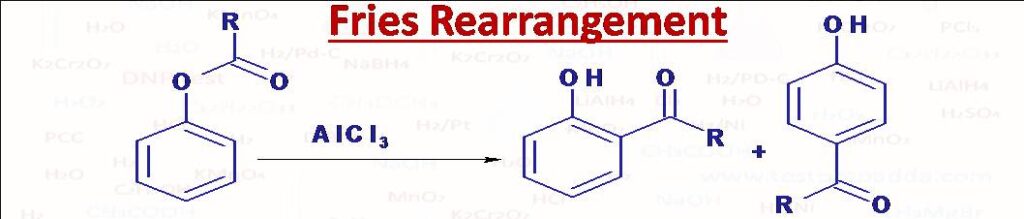
(36) Fries Rearrangement
Phenols (C6H5OH) when treated with acid chlorides (RCOCl) phenyl esters (C6H5OCOR) are formed. These phenyl esters under suitable conditions undergo Fries rearrangement where phenyl ester is converted into ortho or para hydroxyletons or mixture of both by treatment with AlCl3. Low temperatures (60oC or less) favour the formation p- isomer whereas high temperatures (above 160oC) favours the formation of o- isomer.
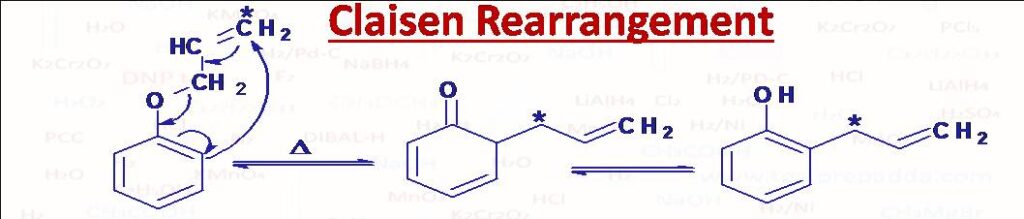
(37) Claisen Rearrangement
Allyl ethers of phenol (C6H5-O-CH2-CH=CH2) when heated at about 200oC rearrange to ortho allylphenols. If both O- positions are occupied then allyl group migrates to para position. Claisen rearrangement can be seen in allyl-vinyl ethers also. Claisen rearrangement is an example of sigmatropic rearrangement (Intramolecular rearrangement in which a sigma bonded atom or group flanked by one or more pi electron systems is shifted to a new location of molecule, the total number of sigma and pi bonds remain unchanged).
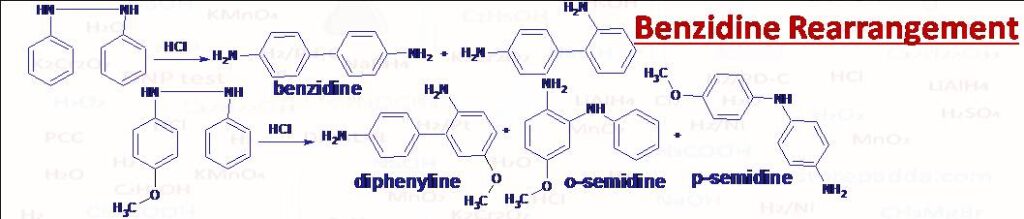
(38) Benzidine Rearrangement
Diphenyl hydrazine (C6H5NH-NH-C6H5) rearranges to 4,4-Diaminodiphenyl (NH2-C6H4-C6H4-NH2) i.e., benzidine when warmed with HCl.If para position of one benzene is occupied then instead of p-p coupling o-p coulpling takes place (Diphenyline) OR o- and p- semidine (Ar-NH-Ar’-NH2) transformations take place according to group attached at para position. If methyl, methoxy type of group is present at p- position the main product will be o-semidine accompanied by small amount of p-semidine. If p- position is occupied by -Cl, -Br, -I or -N(CH3)2 type then main product is diphenyline derivative accompanied by a small amount of o- and p- semidines. All these rearrangements under the influence of HCl or H2SO4 are called Benzidine Rearrangement.
(39) Curtius Rearrangement
Acyl azide RCON3 on heating gives alkyl isocyanate which on hydrolysis gives primary amine RNH2. R group migrates from C to N. This method can be used to convert acid chloride or ester into amine because acid chloride or ester can be converted into acylazide by reaction with first N2H4 to get RCONHNH2 then with HNO2 to get RCO-N=N+=N.
(40) Lossen Rearrangement
This method is for the conversion of acid chloride or ester into amine. First RCOCl or RCOOR’ are converted into RCONHOH (Hydroxamic acid) by reaction with hydroxylamine NH2OH. When this hydoxamic acid RCONHOH is treated with strong inorganic acid like HCl it undergoes a rearrangement called Lossen rearrangement to give alkyl isocyanate RNCO then it is hydrolysed to get RNH2.
(41) Schmidt Reaction (Rearrangement)
There are three reactions called by the name Schmidt reaction involving the addition of hydrazoic acid HN3 to carboxylic acid RCOOH, aldehydes and ketones and alcohols and olefins. The most common one is reaction with carboxylic acid. Sulphuric acid is the most common catalyst used but Lewis acids can also be used. RCOOH when reacted with HN3 in presence of H2/SO4 first we get alkyl isocyanate RNCO then on hydrolysis we get RNH2. Percentage yield is better than Hofmann or curtius rearrangement and good results are obtained for long chain R. Reaction between ketone (RCOR) and HN3 is used to insert NH group between carbonyl C and one R. With alkyl and aryl group it is generally aryl group that migrates from C to N.
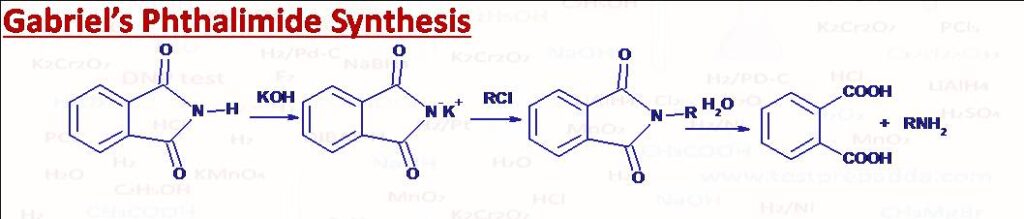
(42) Gabriels Phthalimide Synthesis
It is used for the preparation of pure primary amine (uncontaminated with secondary and tertiary amines). Halides RX are converted into primary amines RNH2 by reacting with potassium phthalimide and then hydrolysis. Aromatic amines can’t be prepared by this method as Aryl halides don’t undergo SN2 reactions.
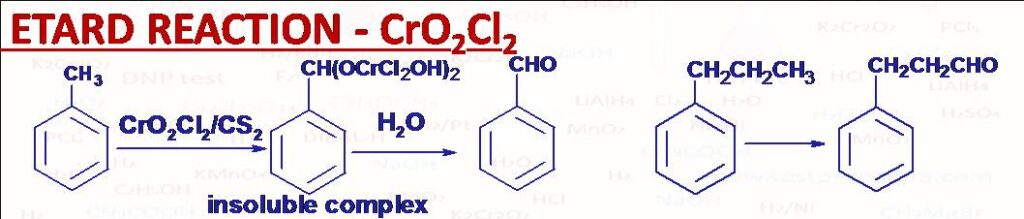
(43) Etard Reaction (CrO2Cl2)
When toluene C6H5-CH3 is treated with chromyl chloride CrO2Cl2 in inert solvents such as CS2 or CCl4 an insoluble complex C6H5-CH(OCrCl2OH)2 forms which on hydrolysis gives benzaldehyde C6H5-CHO. Alkyl benzenes where side chains are larger than methyl (e.g. C6H5-CH2CH2CH3) are oxidised at the terminal carbon (e.g. C6H5-CH2CH2CHO).
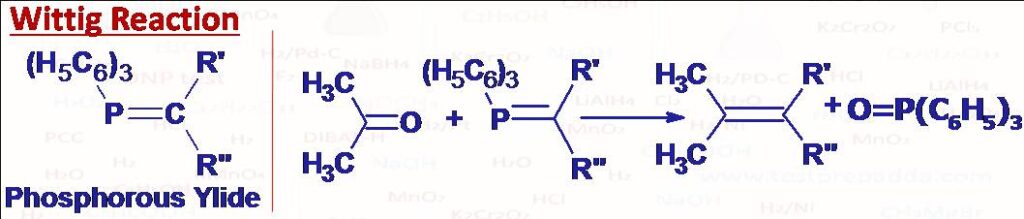
(44) Wittig Reaction
Aldehydes and ketones react with phosphorous ylides (phosphoranes) to give alkenes. This reaction is called Wittig Reaction. An ylide is any compound with opposite charges on adjacent covalently bonded atoms each of which has a complete octet of valence electrons. Since phosphorous can accommodate more than eight electrons in the valence shell, a phosphorous ylide also called phosphorane has an uncharged resonance structure. Phosphorous ylides are formed by SN2 reaction of primary or secondary alkyl halides with triphenylphosphine and then heating with a strong base (like RLi).
(45) Elimination Reactions
A reaction which involves removal of two atoms or groups from same molecule (carbene or nitrene forms). In alpha elimination (1,1) two atoms/groups are lost from same atom, in beta (1,2) elimination two atoms/groups are lost from adjacent atoms of the substrate (multiple bonds form). Dehydration of alcohols (refer reagent H2SO4) and dehydrohalogenation (refer reagent NaOH) are two important examples of beta elimination. Elimination reactions generally follow E1 (first order), E2 (second order), Ei or E<1CB (second order) mechanisms. If reagent is strong base like OH-, C2H5O- it favours E2 and (also favours E1CB if beta H is acidic). For both E1 and E2 reactivity of substrate favours as 3o>2o>1o. Alpha alkyl or alpha aryl groups stabilise the carbocation character of Transition State (T.S) so favours E1, Beta aryl groups favour E1CB. Please note whatever be the mechanism a double bond does not go to a bridge head carbon unless sizes are large enough (8 or more). Newly double bond favours if it is in conjugation with C=C or C=O. In cyclohexane elimination is possible from diaxial conformation, if more stable conformer participates in elimination, then rate will be more. If the substrate has more than one beta H then double bond is formed at different locations. More substituted alkene (Saytzeff or Zaitsev’s product) will be more stable and major in most cases. Least substituted alkene is called Hofmann\’s product. Compounds containing charged nucleofugal leaving group (e.g. -N+R3, -S+R2) follow hoffmann elimination, for alkyl halides Hofmann product increases from I to F but major in F only (due to carbanion character in transition state). Hofmann elimination also increases with increase in branching in base (Me3C-O-).
(46) Substitution Reactions
An atom or group is replaced by another atom or group. Substitution reactions effected by nucleophiles are called nucleophilic substitution (different mechanisms are SN1 i.e., first order, SN2 i.e., second order or SNi i.e., second order. In SN1 carbocation forms as intermediate and product forms with retention as well as inversion (predominant) i.e., partial racemisation. In SN2 reaction nucleophile attacks from backside so inversion occurs in the product but we can’t say if reactant is dextro or R then product can be dextro as well as leavo means R or S depending upon group replaced. For SN2 reactivity of different substrate generally varies as RCOCH2Cl > RNHCH2Cl > ROCH2Cl > C6H5CH2Cl = CH3Cl > CH2=CH-CH2Cl > Me2CHCl > Me3CCl. For SN1 the reactivity generally varies as RNHCH2Cl > ROCH2Cl > Ph2CH-Cl> Me3C-Cl > PhCH2Cl > Me2C-Cl > MeCl. If leaving group (e.g., Halogen) is attached to vinylic carbon or phenyl or bridgehead carbon then reactivity will be low for both SN1 and SN2. Generally weak nucleophiles like H2O, CH3OH favour SN1 and strong nucleophiles like C2H5O- favour SN2. Polar protic solvents like H2O favour SN1 because reactivity of nucleophile decreases and stability of carbocation increases in polar protic solvent by solvation. In many substitution reactions a nucleophilic group within a molecule close to the electrophilic carbon participates temporarily in the reaction to control both rate and stereochemistry. Such involvement of a group is known as neighbouring group participation (NGP) and rate enhancement is known as anchimeric assistance. Halobenzenes do not undergo normal SN1 and SN2 substitution but if they have one or more electron withdrawing groups such as -NO2, -CHO, -CN etc. at ortho or para positions then can undergo substitution under milder conditions (SNAr mechanism) to form ipso product (nucleophile is attached with same carbon from where leaving group leaves) with reactivity order ArF > ArCl > ArBr > ArI. But if aryl halides have no activating electron withdrawing groups then benzyne mechanism is possible with strong base NaNH2, here ipso and cine (substitution at different position – adjacent) products possible with reactivity order ArI > ArBr > ArCl > ArF.
