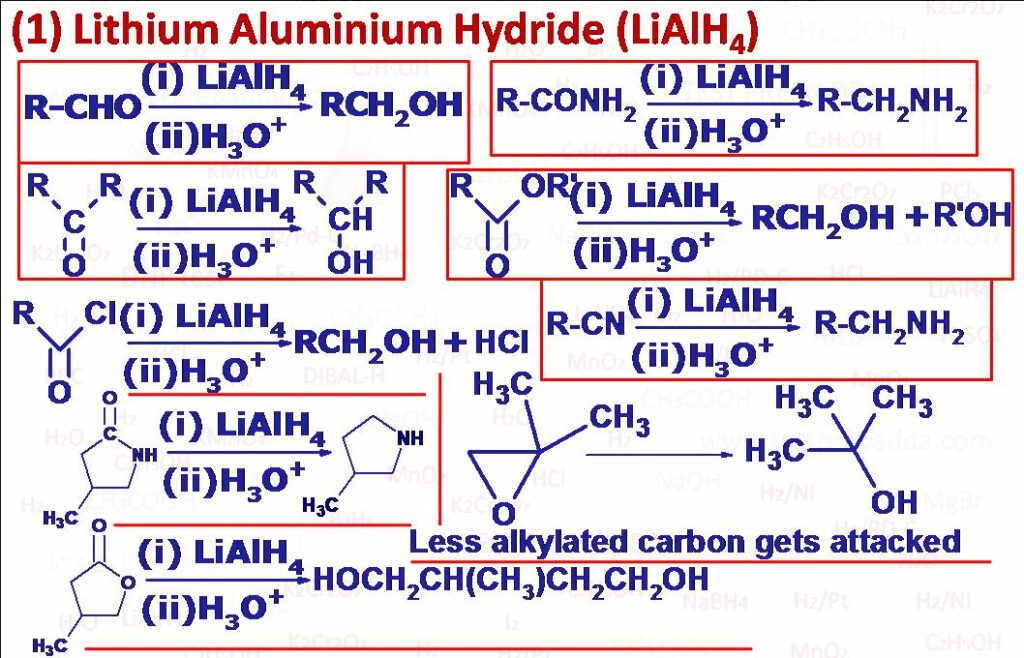
(1) LiAlH4 is mainly used for the reduction of polar unsaturation. Carbon-Carbon double bonds and triple bonds are unaffected due to non-polarity. Aldehydes (RCHO) and Ketones (RCOR) on treatment with Lithium Aluminium Hydride LiAlH4 in dry ether and subsequent acidification yield primary alcohols RCH2OH and secondary alcohols RCH(OH)R’ respectively, for this purpose we can also use NaBH4 but LiAlH4 is a power reducing agent and reduces many other functional groups such as carboxylic acid and its derivatives, nitro compounds. Acid chlorides (RCOOCl) on treatment with Lithium Aluminium Hydride LiAlH4 in dry ether and subsequent acidification yield primary alcohols RCH2OH. Esters (RCOOR) on treatment with Lithium Aluminium Hydride LiAlH4 in dry ether and subsequent acidification yield primary alcohols RCH2OH and ROH. Lactones (cyclic esters) are reduced to corresponding diols. Acid anhydrides (RCO)2O on treatment with LiAlH4 yield primary alcohols RCH2OH. Primary (RCONH2), secondary (RCONHR) and tertiary amides (RCONR2) on reaction with LiAlH4 give primary (RCH2NH2), secondary (RCH2NHR) and tertiary amines (RCH2NR2 respectively (can also be done using H2/Ni) Cyclic amides (Lactams) on reduction give cyclic amines. Nitriles (RCN) are converted into primary amines (RCH2NH2). Isocyanides are converted into N-Methylamines (secondary amines) similar to H2/Ni. Epoxides when treated with LiAH4 in dry ether and subsequent acidification with dilute sulphuric acid gives alcohols. Oximes (>C=NOH), both ketoximes and aldoximes are reduced to primary amines (>CH-NH2Aliphatic nitro compounds are reduced to amines although for aromatic nitro compounds we use metal/HCl not LiAlH4, if we use LiAlH4 > for aromatic nitro compounds ArNO2 we get azo compounds (Ar-N=N-Ar), nitroso compounds can also be reduced to azo compounds. Peroxides are reduced to 2 moles of alcohols. Sulfoxides (RSOR) are reduced to sulphides (RSR). Quinones are reduced to hydroquinones by LiAlH4 as well as SnCl2/HCl
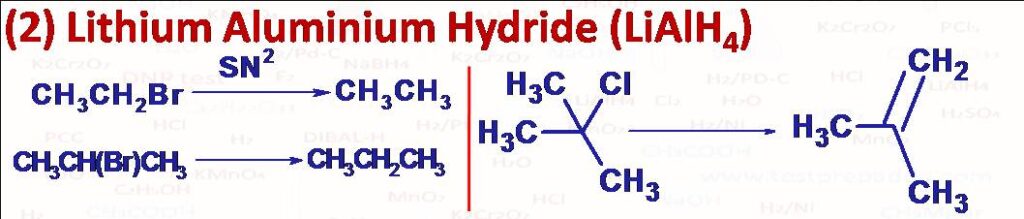
(2) Primary and secondary alkyl halides and alkyl sulphonates are readily reduced to corresponding alkanes by treatment with LiAlH4 in dry ether or THF solvent. Tertiary alkyl halides and sulphonates mainly undergo elimination reaction to give alkenes.
