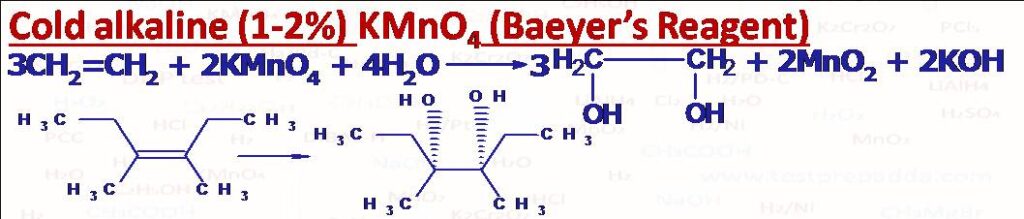
(1) Cold alkaline (1%) KMnO4 (Baeyer’s Reagent): When alkenes are treated with dilute alkaline (1 or 2%) KMnO4 at room temperature vicinal glycols (adjacent carbon atoms having -OH groups are obtained). This addition occurs in syn manner (concerted one step). cis-But-2ene gives meso-2,3-Butane-diol and trans-But-2-ene gives Racemic mixture of 2,3-Butanediol. The reaction is Stereospecific. This solution has purple (or lilac) colour and mainly used to test whether compound has double and triple bonds or not. After the reaction the colour disappears and brown precipitate of MnO2 forms. Same vicinal glycols can be obtained using osmium tetroxide (OsO4), in this reaction cyclic osmate ester forms like cyclic manganate ester in KMnO4 then by hydrolysis with aqueous sodium sulphite or bisulphite we get vicinal glycol, this is also a syn addition.
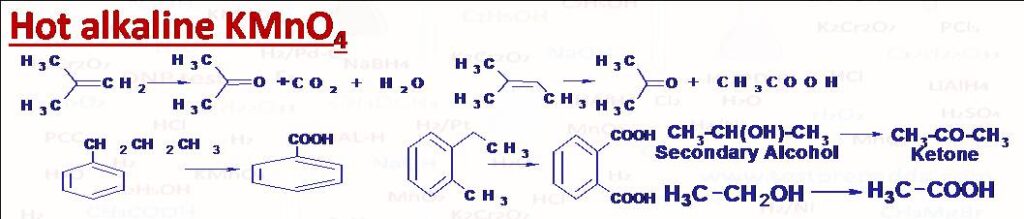
(2) Hot alkaline KMnO4: (i) Conversion of alkene into acid and/or ketone. Please note that double bond carbon which bears one hydrogen atom only gets converted into -COOH group, and double bond carbon atom which bears two H atoms gets converted into CO2 and H2O, and the double bond carbon which bears no H gets converted into ketone >C=O. (ii) Conversion of primary alcohol into acid and secondary alcohol into ketone. (iii) Conversion of alkyl benzene into aromatic acid (C except 3o directly attached with benzene ring is converted into –COOH). In case of vigorous oxidation like heating with acidic KMnO4 even ketones break predominantly between carbonyl carbon and alpha carbon of larger alkyl, this rule is called Popov’s rule.
