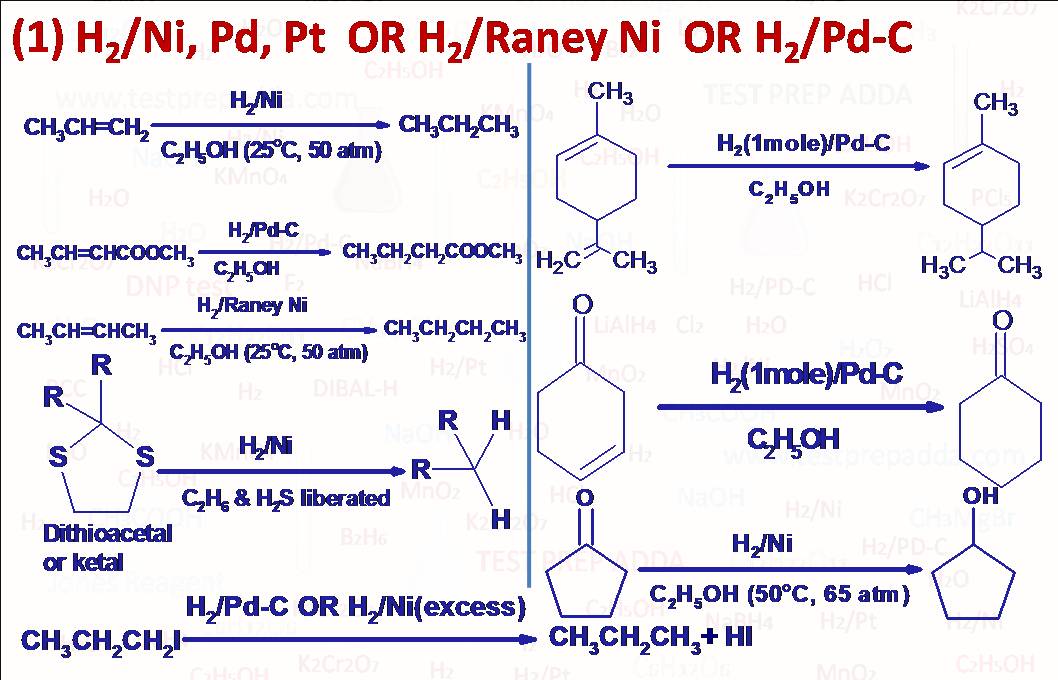
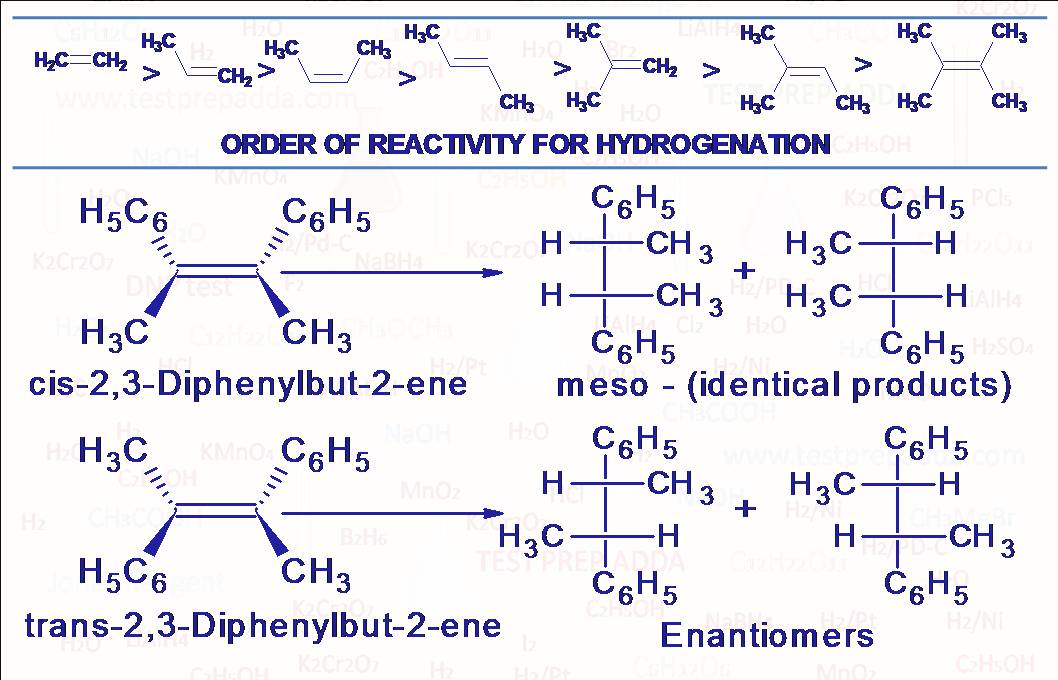
(1) Alkenes and alkynes react with hydrogen (H2) in the presence of metal catalyst such as Nickel (Ni), Palladium (Pd) and Platinum (Pt) to produce alkanes. More stable alkene means less reactivity for hydrogenation and less heat evolved during hydrogenation (Heat Of Hydrogenation). This catalytic reduction is generally used for reduction of nonpolar unsaturations (alkene, alkyne) but can also be used for reduction of polar unsaturation (aldehyde, ketone, nitrile etc.) but it requires strenuous conditions (heat and pressure). Please note that a carbon-carbon unsaturation is more reactive towards this catalytic hydrogenation than other functional groups such as aldehyde, ketone, nitrile, acid, ester etc. This catalytic hydrogenation is sometimes regioselective (with insufficient hydrogen) for less substituted carbon-carbon double bond in presence of more substituted double bond. Pt is generally used as (PtO2)(Adam\’s catalyst), In place of Ni we may use Raney Ni for better producibility. Another important point is that addition is syn addition so this catalytic hydrogenation is also STEREOSELECTIVE and STEREOSPECIFIC, it means cis-alkene (symmetrical) on hydrogenation may give meso compound and trans-alkene may give enantiomers (d,l). The dithioacetal ot dithioketal when hydrogenated over Raney Ni give corresponding alkane (used in Mozingo reduction). Primary, secondary and tertiary alkyl iodides, tertiary alkyl bromides and chlorides are converted into alkanes. Each double bond consumes one molar equivalent of hydrogen, each triple bond consumes two while rings are unaffected by hydrogenation at room temperature but please note that each ring equates with one unit of hydrogen deficiency also called degree of unsaturation (The number of pairs of hydrogen atoms that must be subtracted from the molecular formula of corresponding alkane to give the molecular formula under consideration). For organic compounds containing C, H, N, X, and O, The Degree Of Unsaturation = Number of carbon atoms + 1 – (Number of H atoms/2) – (Number of halogen atoms/2) + (Number of N atoms/2), please note that one double bond is equivalent to one degree of unsaturation, one triple bond is equivalent to two degree of unsaturation and one ring is equivalent to one degree of unsaturation. Cyclopropane can be cleaved by catalytic hydrogenolysis to give propane even under mild conditions. Benzene shows some of addition reactions like alkene and alkyne under more drastic condition to form addition products. It has four degree of unsaturation (3 double bonds and one ring). The addition products are stable and behave as saturated hydrocarbons. Hydrogenation of benzene takes place in the presence of catalyst like nickel or palladium at 475 to 500K temperature to give cyclohexane. Hydrogenation can be used to convert a nitro compound to amine but not used when the molecule contains some other easily hydrogenated group like carbon-carbon double bond.
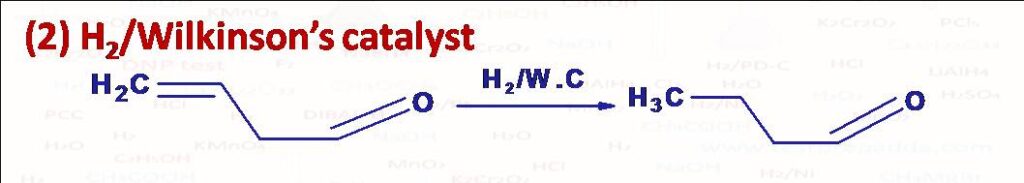
(2) Hydrogenation is considered to be heterogeneous with Ni, Pt, Pd, Raney Ni, Rhodium, Ruthenium etc. because catalysts are insoluble in reaction medium. Wilkinson’s catalyst chlorotris(triphenylphosphine)rhodium, RhCl(Ph3P)3 is soluble in reaction medium so with this hydrogenation is homogeneous hydrogenation and this is highly regioselective and reduces olefinic compounds(-C=C-)without disturbing groups such as -COOR, -NO2, -CN, -COR present in the same molecule. Another homogeneous catalyst is tris(triphenylphosphene)hydridoruthenium(II) which is specific for terminal double bonds (others may or may not get reduced). One more is Pentacyanocobaltate(II) for double and triple bonds only when these are part of conjugated system (conjugation can be with >C=O, C=C, or aromatic ring). Homogeneous catalysts have advantage of better selectivity and reproducibility over heterogeneous.
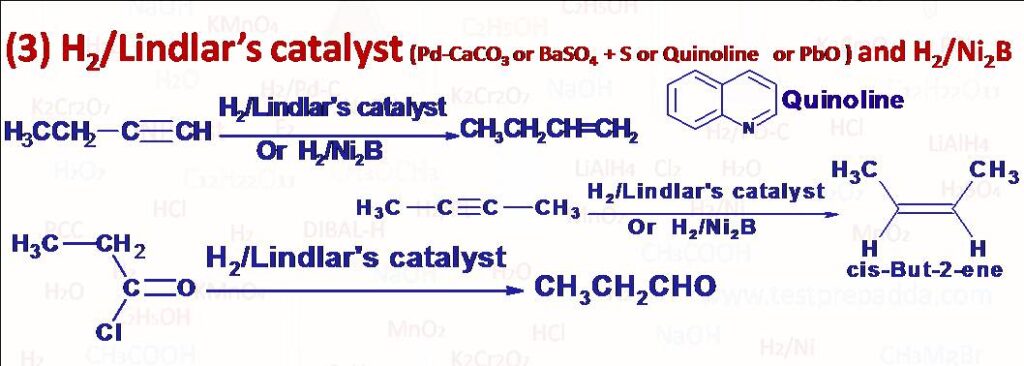
(3) Controlled reduction of alkynes with H2 gas in the presence of partially deactivated catalyst Lindlar’s Catalyst gives alkenes. Lindlar catalyst consists of palladium deposited in the finely divided state on a solid support such as CaCO3 or BaSO4 which is poisoned or we can say partially deactivated with either Sulphur, Quinoline, Lead acetate or Lead oxide. Non-terminal alkynes are converted into cis alkene due to syn addition. For the same purpose i.e. controlled hydrogenation of alkynes we can use another catalyst called P-2 catalyst Nickel boride (Ni2B). Nickel boride is prepared by reduction of Nickel acetate with NaBH4 Sodium borohydride). Another use of Lindlar’s catalyst is preparation of aldehydes from acid chloride. Acid halides are reduced to aldehydes when treated with H2 gas in boiling Xylene in the presence of Lindlar’s catalyst.

(4) With H2/CuO.CuCr2O4 generally esters are reduced to alcohols and carboxylic acid is also reduced to primary alcohol.
