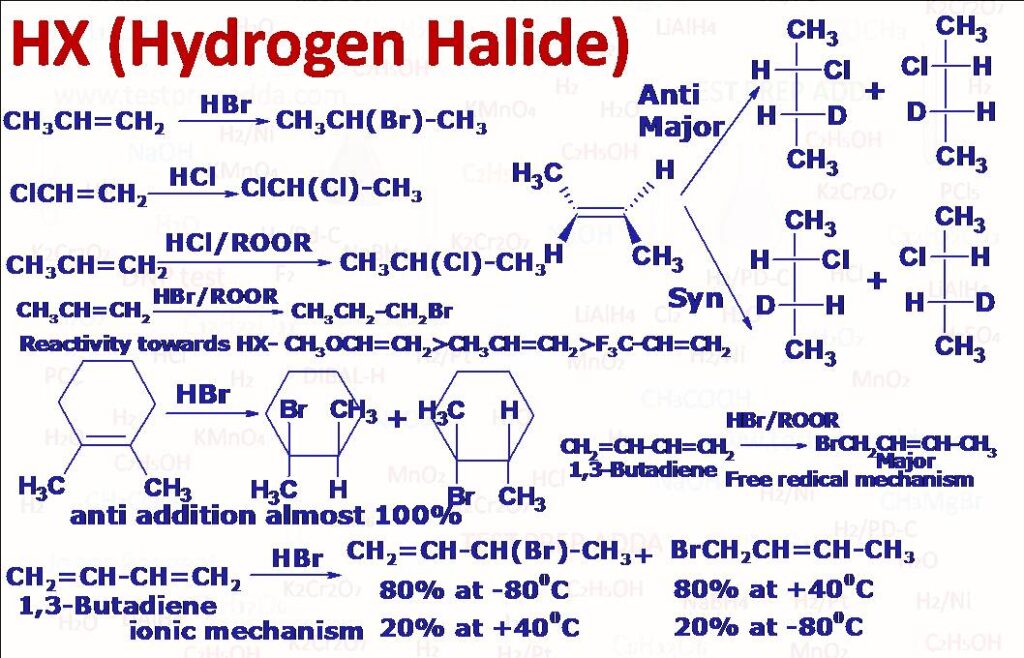
(1) ADDITION OF HX TO ALKENE: Dry gaseous hydrogen halide (not aqueous) is used to avoid the formation of alcohol. Alkene itself can act as solvent but sometimes we can use solvents like CH2Cl2. Reactivity order for hydrogen halides is HI>HBr>HCl>HF. Reaction follows ionic mechanism (via carbocation: whenever possible rearrangement takes place-1,2 hydride shift or 1,2 alkyl shift or ring expansion) so more stable carbocation formed by alkene implies more rate of the reaction, the addition mainly takes place by anti-addition predominantly (although in many cases syn product also forms in sufficient amount) . When HBr is added to alkene in the presence of peroxide like R-O-O-R reaction follows free radical mechanism and addition takes place with Anti-Markovnikov rule (Peroxide effect or Kharasch and Mayo effect), This effect is not shown by other hydrogen halides HF, HCl and HI. If we add few inhibitors like dihexylamine or hydroquinone then they will trap the free radical and there will not be any peroxide effect with HBr even though peroxide is present.
(2) ADDITION OF HX TO ALKYNE: Alkynes first give vinyl halides then on addition of another molecule of HX we get geminal dihalides. Addition takes place according to Markovnikov\’s addition. Vinyl carbocation is less reactive than alkyl carbocation so mostly alkenes react faster than alkynes. The addition of HX to vinylhalide occurs less readily than that of alkyne so reaction can be stopped at vinyl halide stage. In the presence of peroxide addition of HBr (not with HF, HCl, HI) takes place by Antimarkovnikov\’s addition. On hydrolysis these geminal halides give aldehydes and ketones.
(3) ADDITION OF HX TO CONJUGATED ALKADIENE: Mixture of 1,2-addition and 1,4-addition products forms and reaction follows ionic mechanism. 1,2-addition product (called kinetically controlled product) forms at low temperature (around -80oC) and 1,4-addition product (called thermodynamically controlled product) forms at higher temperature (around +40oC). In the presence of peroxide free radical mechanism is followed and we get mixture of 1,2 and 1,4 addition products (similar products are obtained when conjugated alkadienes are treated with Br-CCl3, -CCl3 gets attached at 1st carbon).
