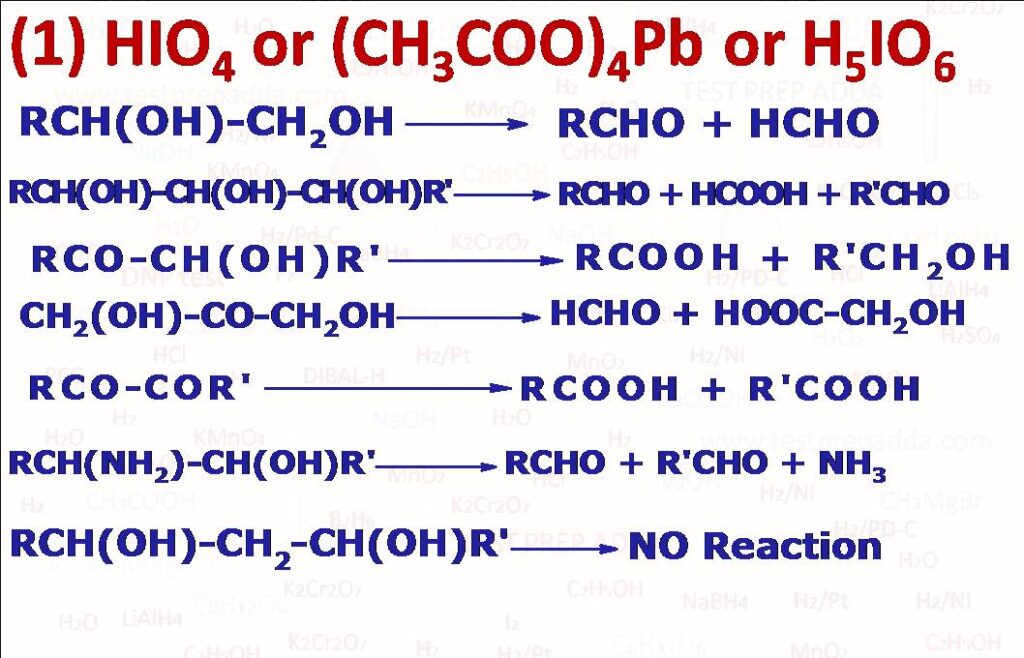
(1) Compounds containing two or more -OH or O groups attached adjacent carbon atoms upon treatment with periodic acid HIO4 undergo oxidation with cleavage of carbon-carbon bonds. -CHO group is oxidised to formic acid, -CH2OH to formaldehyde, >CHOH to aldehyde or formic acid according to the positions of the groups. The reagent does not react with 1, 3 diols or 1, 4 diols or carbonyl compounds. HIO4 is reduced to HIO3. The oxidation is useful in determining the structure. For the same bond cleavage, we can use Lead tetra acetate (CH3COO)4Pb. The two reagents HIO4 and (CH3COO)4Pb are complementary since periodic acid is best used in water and lead tetra acetate in organic solvents. Similar cleavage is undergone by other compounds that contain oxygens and nitrogens on adjacent carbons beta-aminoalcohols, alpha-hydroxyaldehydes and ketones, alpha-Diketones, aldehydes Glyoxals. By HIO4 we can convert epoxides into aldehydes. Alpha hydroxy acids and alpha keto acids are not cleaved by HIO4 but are cleaved by lead tetra acetate. alpha-hydroxyacids give aldehydes or ketones and alpha-ketoacids give carboxylic acids.
