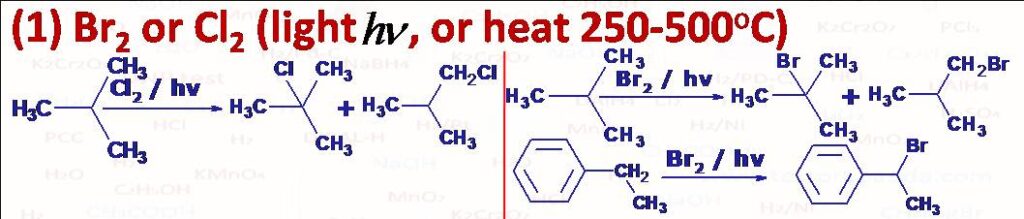
(1) Cl2 or Br2 in presence of sunlight, ultraviolet light or heat at high temperature 250 to 500oC react with alkanes to form a mixture of mono and poly halo alkanes by successive replacement of the hydrogen atoms form alkanes. The reactivity order is F2 >> Cl2 > Br2 >> I2. Since F2 is so reactive it is difficult to control the reaction so we normally don’t use fluorination. I2 is least reactive and reaction is reversible so to carry out the iodination we use some oxidising agents such as HIO3, HNO3, HgO etc. These halogenations take place with free radical mechanism (Chain reaction). For monohalogenation H can be replaced with halogen from tertiary carbon, secondary carbon and primary carbon atoms and at the same time if in the product any of carbon becomes chiral then with respect to that we one more stereoisomer (enantiomer with respect to that carbon). Alkanes which contain different types of hydrogen atoms (primary, secondary and tertiary hydrogen atoms) the selectivity is in the order benzyl, allyl > tertiary H > secondary H > primary H. Bromine is far more regioselective than chlorine even though it is less reactive than chlorine. In chlorination the relative reactivity for the tertiary, secondary and primary H atoms is 3o:2oH:1oH = 5:3.8:1 (at 250C). and In bromination the relative reactivity for the tertiary, secondary and primary Hydrogen atoms is 3o:2oH:1oH = 1600:82:1 (at 1270C). Chlorination can also be effected with sulphuryl chloride (SO2Cl2) in the presence of light (hv) or free radical initiator like peroxide. NBS can also be used for bromination as it supplies low concentration of Br (especially for allylic and benzylic positions).
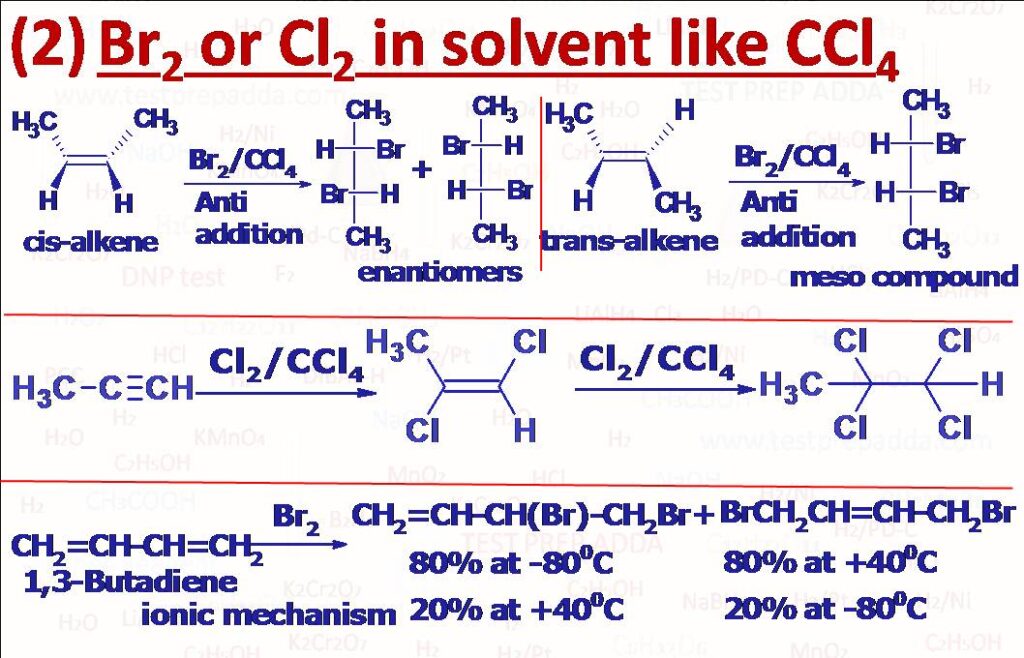
(2) Cl2 or Br2 in an inert solvent such as CCl4 react with alkenes to give vicinal dihalides. Order of reactivity for halogens is F2 >> Cl2 > Br2 >> I2. Order of reactivity for alkenes is (CH3)2C=CH2 > CH3CH=CHCH3 > CH3CH=CH2 > CH2=CH2. Products are formed by anti-addition. These reactions are stereospecific and stereoselective. cis-But-2-ene gives Racemic mixture of 2,3-Dihaloutane while trans-But-2-ene gives meso-2,3-Dihalobutane. In the reaction cyclohalonium ion forms as an intermediate. Please refer Hunsdiecker Reaction from named reactions. Cl2 and Br2 are capable of replacing one or more alpha hydrogen atoms of aldehydes (RCH2CHO) or ketones (RCOCH2R) in acid or base catalysed reactions to form RCH(Cl)CHO or RCOCH2Cl, SO2Cl2 can also be used. Alkynes react with halogens (Cl2 or Br2) in an inert solvent to give first trans-dihaloalkenes then on addition of one more molecule of X2 vicinal tetrahaloalkanes. Buta-1,3-diene on reaction with one mole of Br2 or Cl2 forms two products 1,2-addition product (major at low temp around -80oC) and 1,4-addition product due to resonance (major at high temp around 40oC). 1,4-addition product is called thermodynamically controlled product while 1,2-addition product is called kinetically controlled product (KCP).
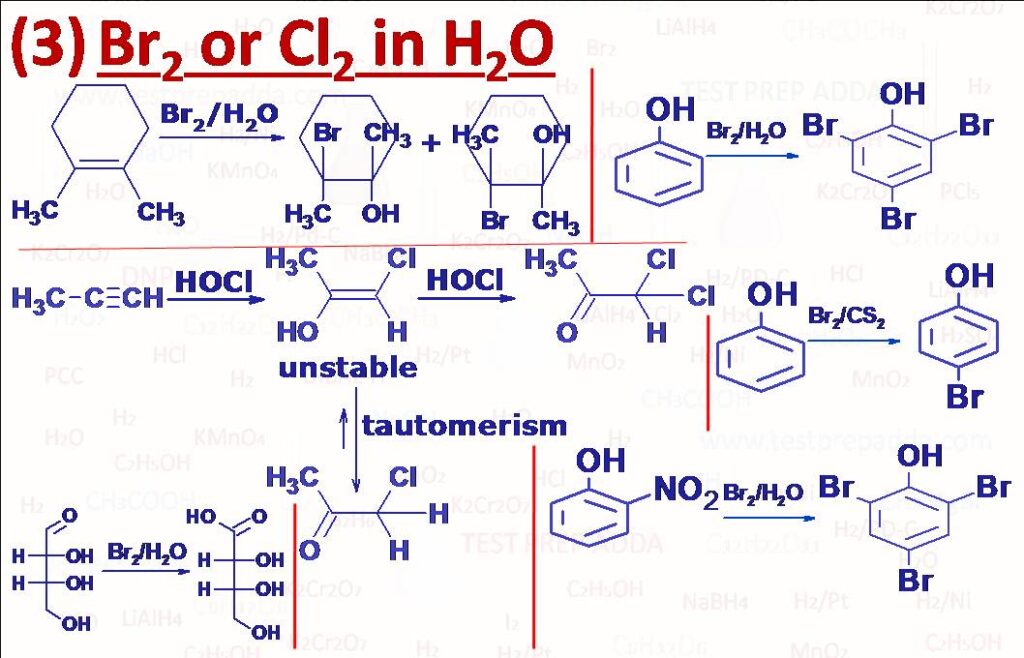
(3) Br2/H2O or Cl2/H2O: ALKENES react with a solution of halogen in water to form halohydrins (compounds that contain a halogen and a hydroxy group bonded to adjacent carbon atoms. The net result is the addition of components of HOX (OH negative part and X positive part). Addition takes place in accordance to Markovnikov’s rule. The reaction is stereoselective as it involves only anti addition. Acetylene reacts with HOCl to form dichloroacetaldehyde and other ALKYNES react as per Markovnikov’s addition to form 1,2-dichloroalkanone. PHENOL on treatment with chlorine or bromine water gives an immediate precipitate of the 2,4,6-trihalogen derivative, if gaseous Cl2 or Br2 attacks the phenol at around 160oC mainly o-halogen derivative is formed, p-bromophenol is prepared by adding Br2 in CS2, please note if phenol has any negative group at o, p such as -NO2, -COOH, -SO3H on treatment with aqueous halogens (Br2/H2O) negative group is often displaced.
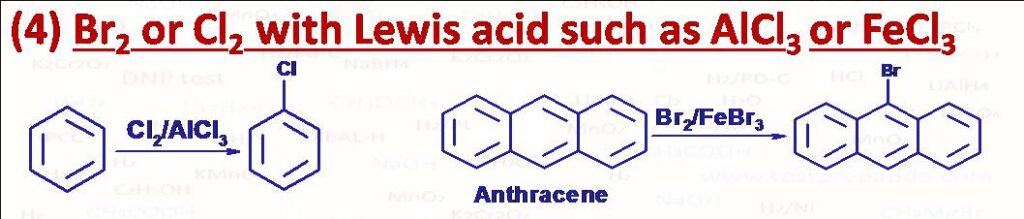
(4) X2 (Br2 or Cl2) / AlX3 (or any Lewis acid such as FeX3, BF3 etc.): This reagent is used for halogenation of benzene ring (electrophilic aromatic substitution – EAS). H atom of benzene ring gets substituted by halogen atom. Fluorine (F2) is highly reactive and mono fluorination is carried out with AgF2 or XeF2. Iodine is unreactive but iodination can be carried out in the presence of an oxidising agent such as HNO2, H2O2. Bromination (addition or substitution) of Anthracene or Phenanthrene takes place at 9-position.
