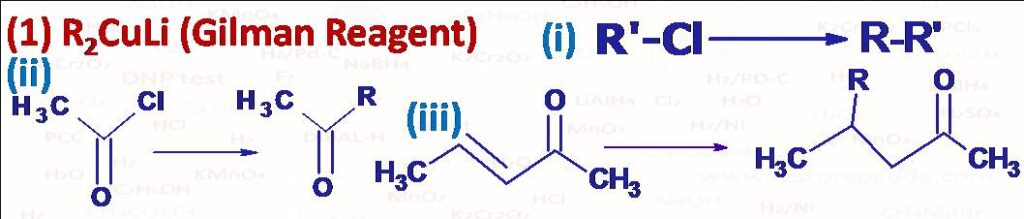
(1) It converts alkyl halides into alkane, acid chloride into ketone (for this R2Cd can also be used prepared by reacting RMgX with CdCl2) and reacts with alpha-beta unsaturated aldehydes and ketones predominantly by 1,4 additions.

(2) Preparation of alkane using Gilman Reagent is known as Corey House Synthesis, and is a versatile method for preparing both symmetrical and unsymmetrical alkanes from alkyl halide in good yield. To prepare Gilman reagent first alkyl halide is treated with Li metal in dry ether or T.H.F solvent at 0oC to form alkyllithium (RLi), which when treated with Cuprous iodide to form lithium dialkylcuperate (R2CuLi), Gilman Reagent. The reactivity order R-I>R-Br>R-Cl. This Gilman reagent gives symmetrical alkane when treated with same alkyl halide R-X (R-R) and gives unsymmetrical alkane (R-R) when treated with different alkyl halide (R-X). For good yield R-X should be primary alkyl halide while R-X can be primary, secondary as well as tertiary. If R-X is tertiary then it undergoes elimination reaction instead of coupling and gives alkene.
