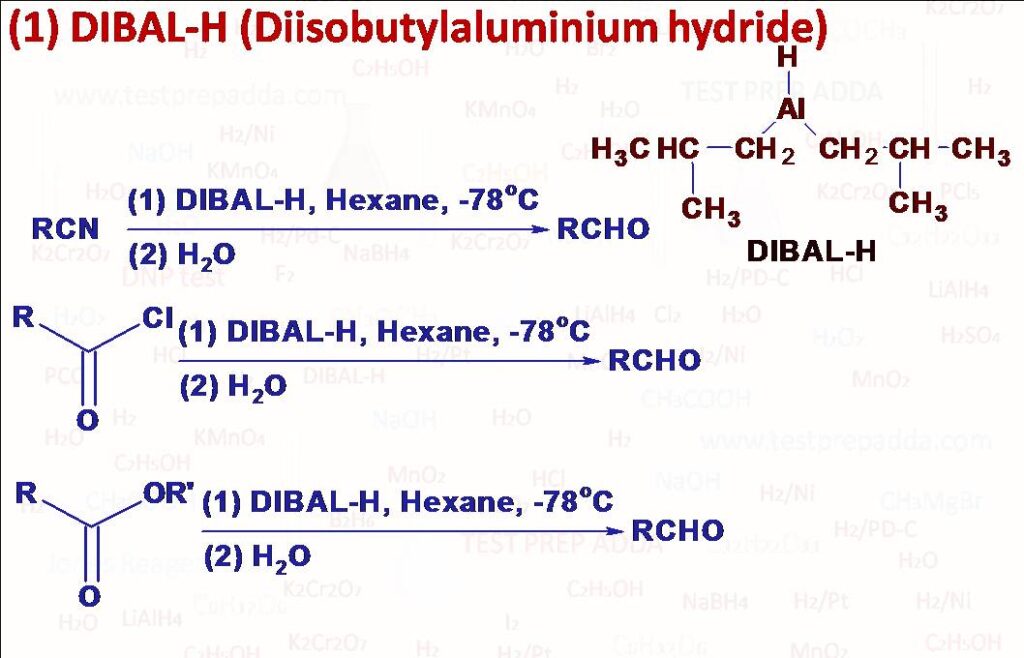
(1) Two derivatives of aluminium hydride that are less reactive than LiAlH4 are Lithium tri-tert-butoxyaluminium hydride and Diisobutylaluminium hydride (DIBAL-H) because these are much more sterically hindered and therefore have difficulty in transferring hydride ions. These reagents are used to convert acid derivatives like acid chloride, ester, nitrile etc. into aldehyde. LiAlH4 can reduce oximes (>C=NOH), both ketoximes and aldoximes to primary amines (>CH-NH2) but DIBAL-H reduces oximes (RRC=N-OH) to secondary amines (R-NH-CH2-R’) arising from a rearrangement. Triple bonds can also be selectively reduced to double bonds.
