DAILY POLL QUESTIONS- SOLUTIONS
Date: 19-07-2022
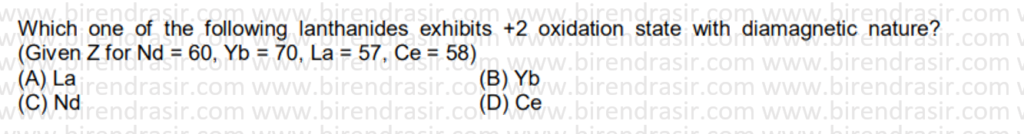
Answer B
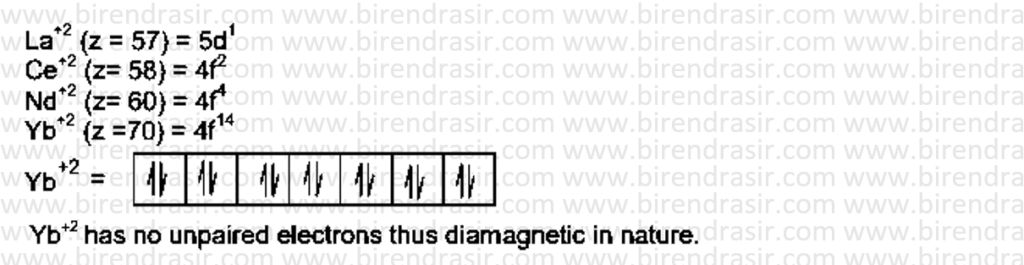
Date: 18-07-2022

Answer B

Date: 17-07-2022

Answer A

Date: 16-07-2022
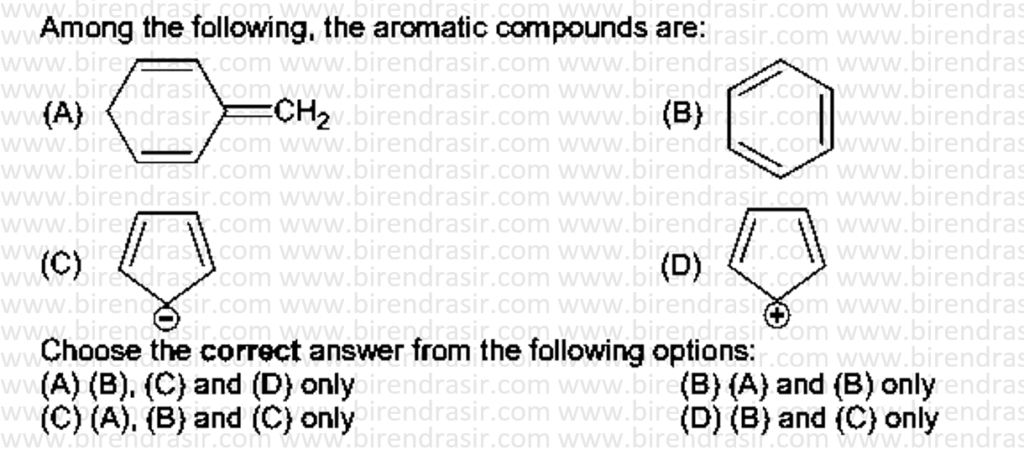
Answer D

Date: 15-07-2022

Answer B

Date: 14-07-2022

Answer C

Date: 13-07-2022

Answer B

Date: 12-07-2022

Answer C
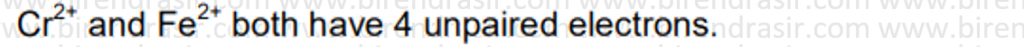
Date: 11-07-2022
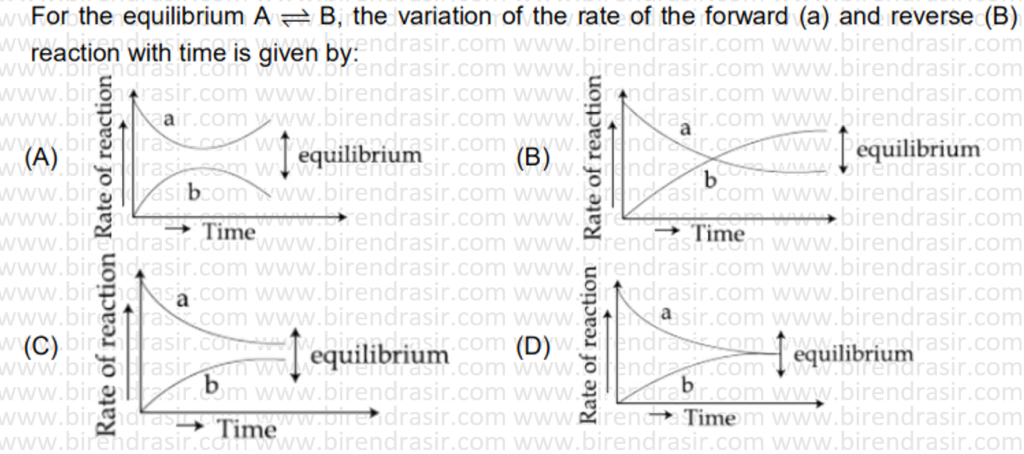
Answer D

Date: 10-07-2022
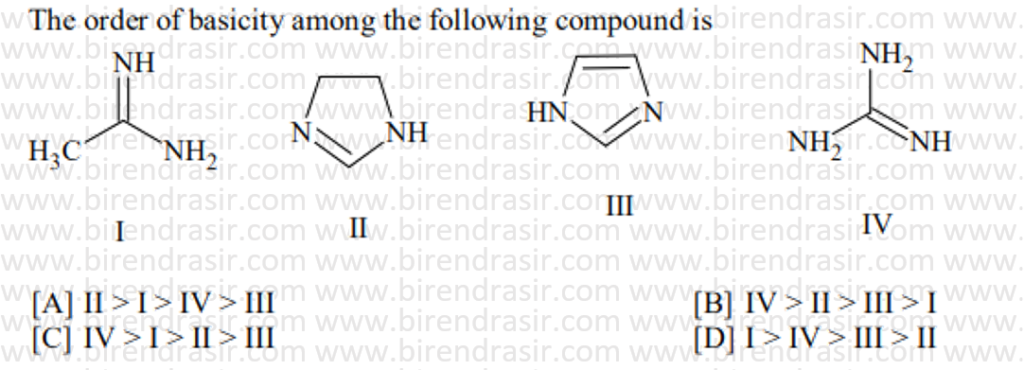
Answer C
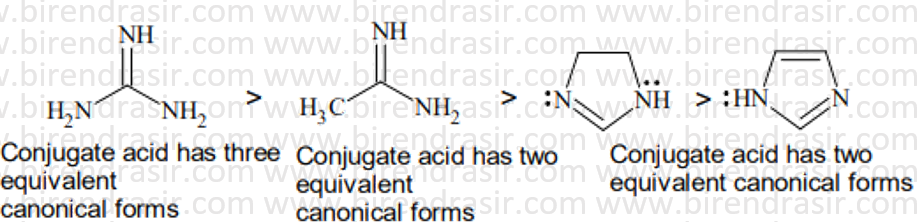
Date: 09-07-2022

Answer B

Date: 08-07-2022

Answer C

Date: 07-07-2022

Answer D

Date: 06-07-2022

Answer B

Date: 05-07-2022
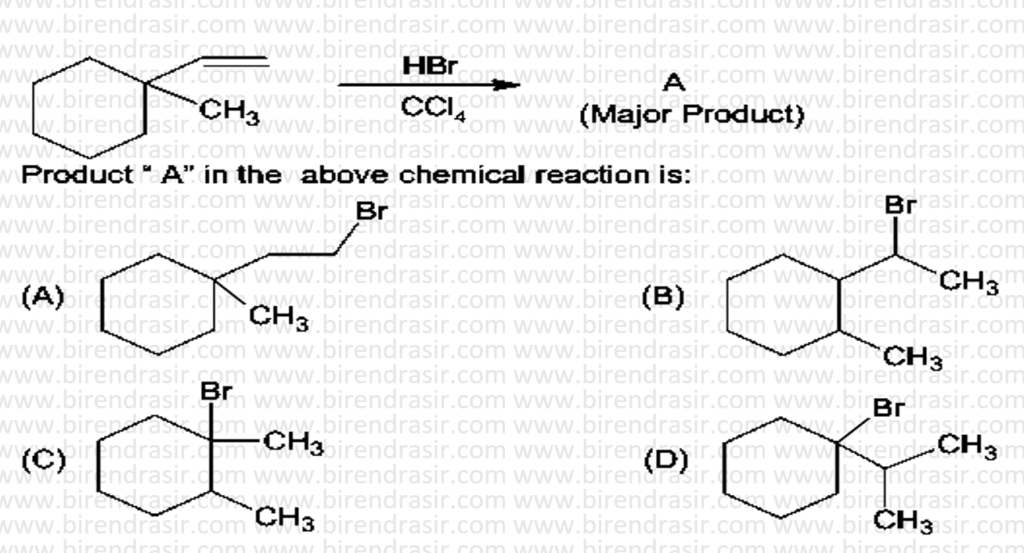
Answer D
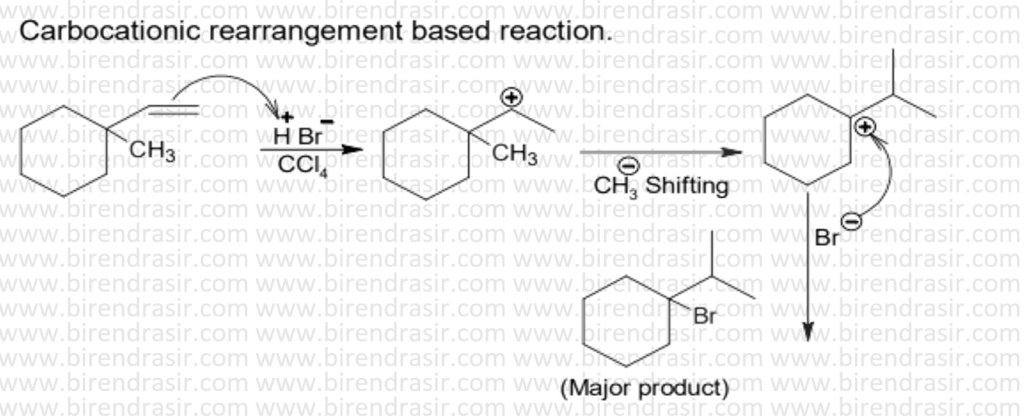
Date: 04-07-2022

Answer C
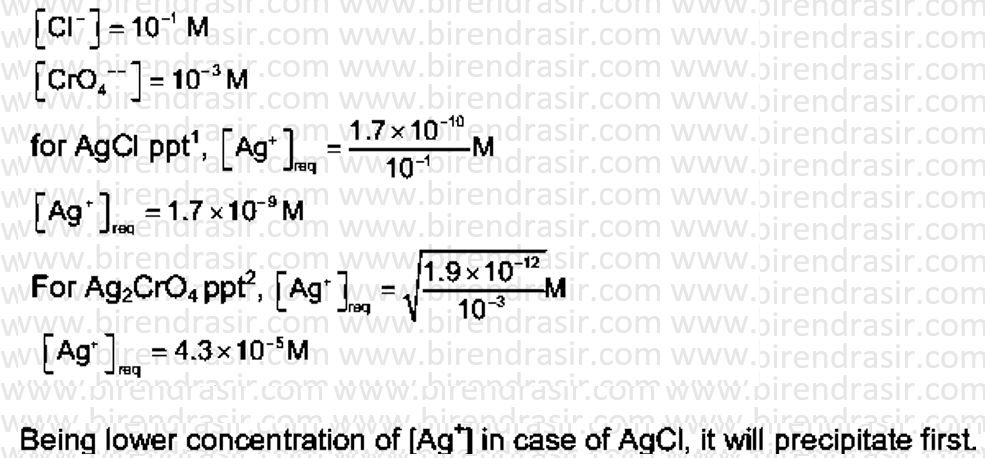
Date: 03-07-2022

Answer A
Basic strength here is decided by electron density on nitrogen. More electron density on N implies more basic compound. Electron withdrawing group decreases basic strength while electron releasing group increases basic strength. In A two CH3CO groups electron withdrawing groups via resonance will decrease the basic strength maximum.
Date: 02-07-2022
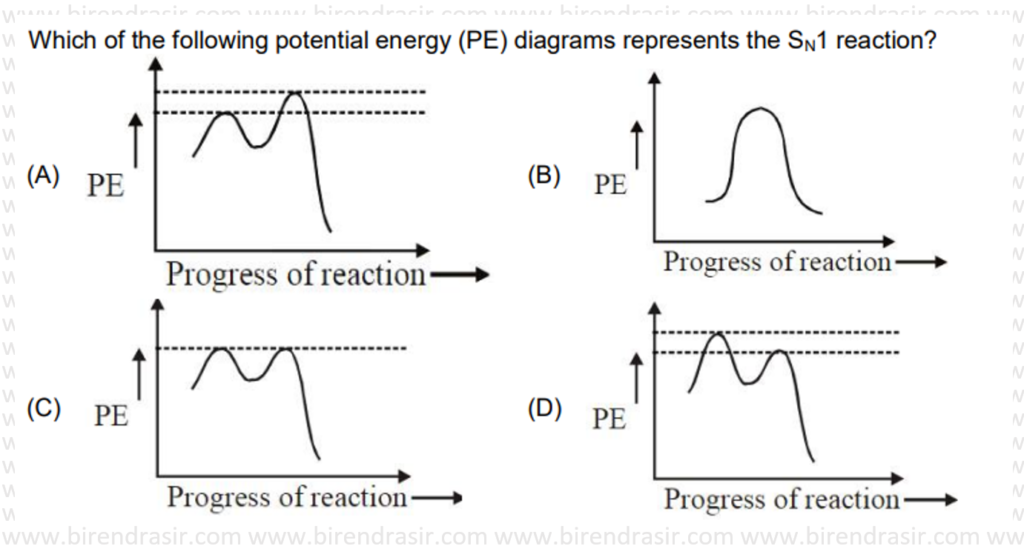
Answer D
SN1 reaction follows carbocation mechanism, where first step is rate determining step i.e. formation of carbocation.
Date: 01-07-2022
Q: Atomic radius of Ga is less than Al
(A) True
(B) False
Answer A
Atomic radius of Ga is less than Al is due to difference in the inner core of the electronic configuration. Presence of additional 10 d electrons offer poor screening effect for outer electrons from the increased nuclear charge in gallium as a result atomic radius of gallium is less than aluminium.
Date: 30-06-2022

Answer – C
Date: 29-06-2022
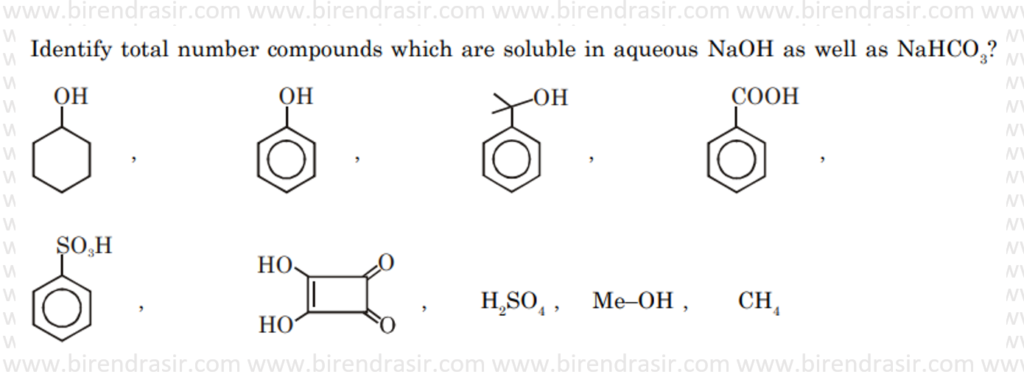
Answer – 4

Date: 28-06-2022
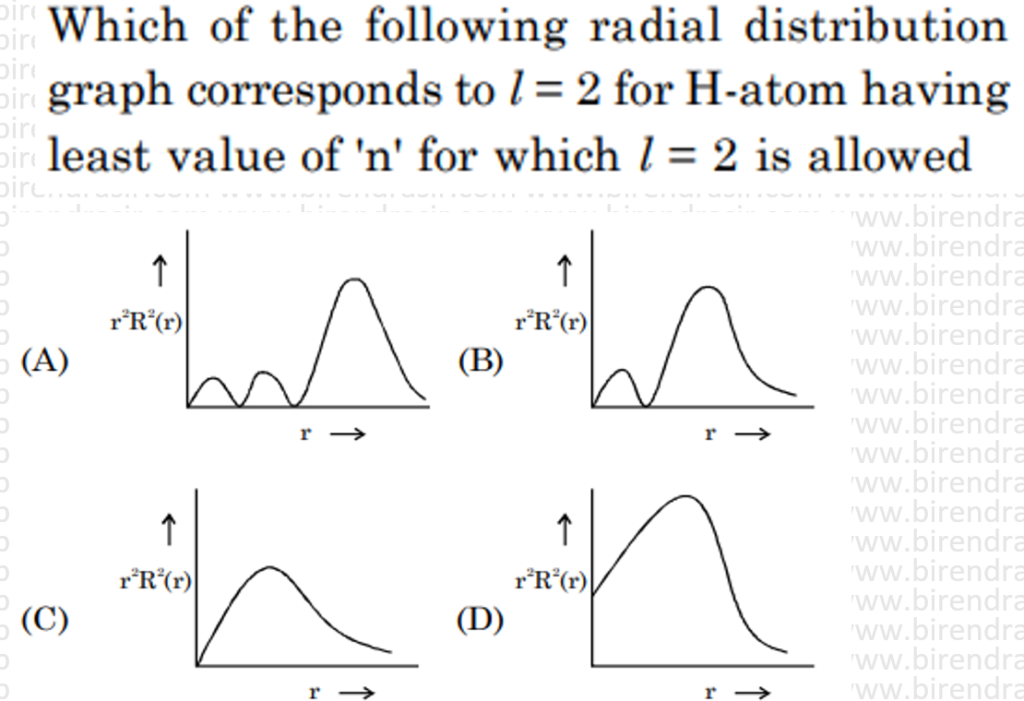
Answer – C
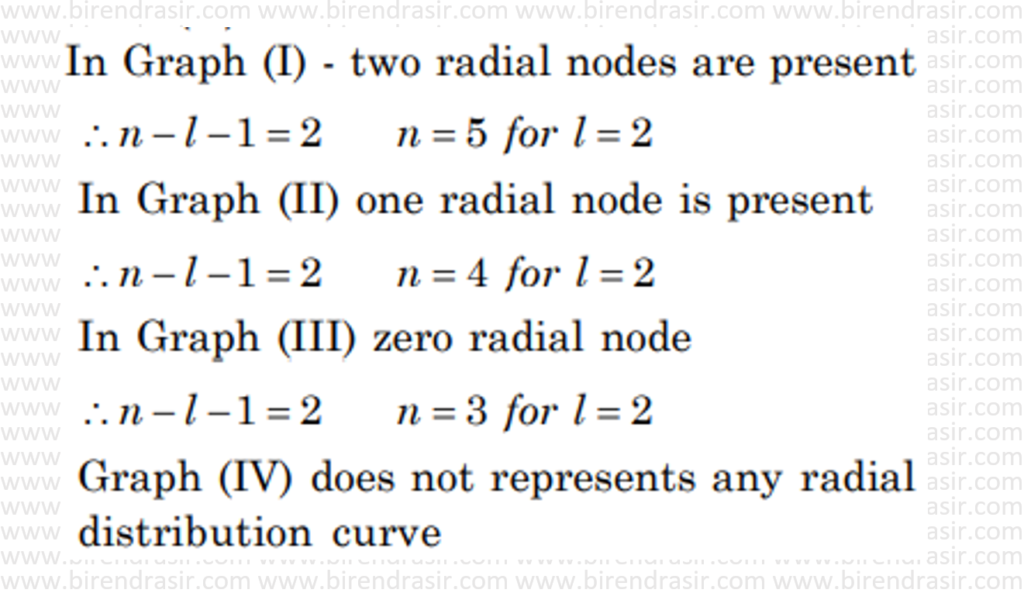
Date: 27-06-2022

Answer – B
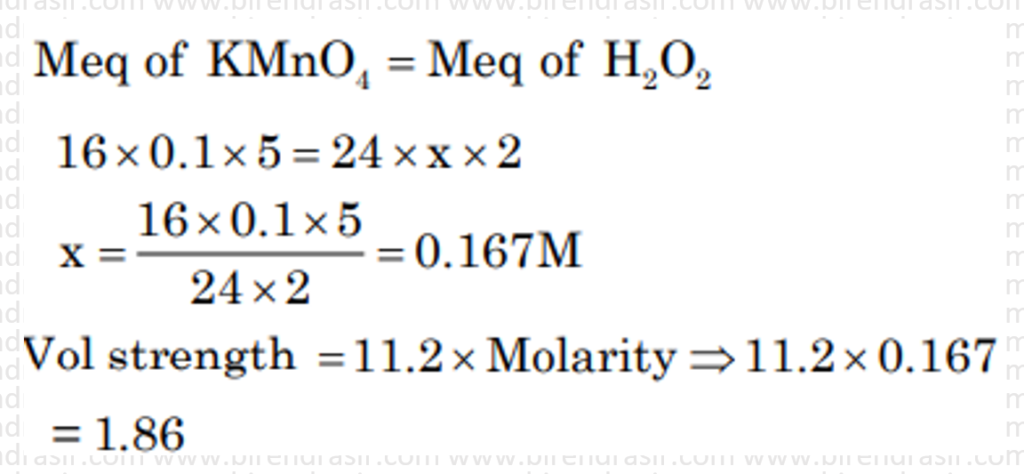
Date: 26-06-2022
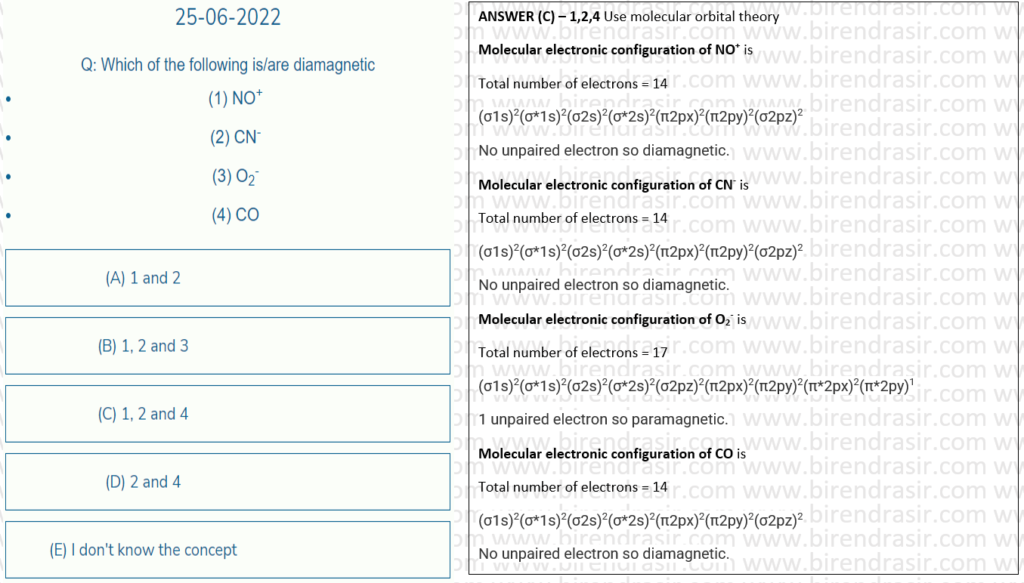
Date: 25-06-2022
Date: 24-06-2022
Date: 23-06-2022
Date: 22-06-2022
Date: 21-06-2022
Q: Number of optically active stereoisomers possible for butane-2,3-diol is
(A) 1
(B) 2
(C) 3
(4) 4
Date: 20-06-2022
Q: 2-Methylaniline is more basic than 4-Methylaniline.
(A) True
(B) False
Date: 19-06-2022
Q: Pyridine is more basic than Pyrole.
(A) True
(B) False
Date: 18-06-2022
Q: o-Nitrophenol is more acidic than m-Nitrophenol.
(A) True
(B) False
Date: 17-06-2022
Q: if a liquid and its vapour are at equilibrium and the pressure is suddenly decreased then
(A) heating occurs
(B) cooling occurs
(C) no effect.
Date: 16-06-2022
Q: All the Al-Cl bonds in Al2Cl6 are equivalent.
(A) True
(B) False
Date: 15-06-2022
Q: Rate constant of a reaction depends on
(A) time of reaction.
(B) extent of reaction.
(C) initial concentration of reactant
(D) temperature
Date: 14-06-2022
Q: What is the molality of 3 M NaCl solution whose density is 1.25 g/ml?
(A) 3
(B) 1.56
(C) 2.79
(D) 3.56
Date: 13-06-2022
Q: N(SiMe3)3, Tris(trimethylsilyl)amine and NMe3, Trimethylamine are isostructural.
(A) True
(B) False
Date: 12-06-2022
Q: The maximum possible number of hydrogen bonds a water molecule can form is
(A) 1
(B) 2
(C) 3
(D) 4
Date: 11-06-2022
Q: The heat absorbed during isothermal expansion of an ideal gas against vacuum is zero.
(A) True
(B) False
Date: 08-06-2022
Q: What is the bond order of carbon monoxide ion (I), CO+ ?
(A) 3
(B) 3.5
(C) 2.5
Date: 07-06-2022
Q: Which of the following statements is incorrect?
(A) The first ionisation potential of Na is less than Mg.
(B) The first ionisation potential of Al is less than Mg.
(C) The second ionisation potential of Mg is greater than Na.
Date: 26-05-2022
Q: CYCLO HEXANE IS MORE STABLE THAN BENZENE.
(A) True
(B) False
Date: 25-05-2022
Q: If number of isomeric benzene derivatives of molecular formula C8H10O, which can liberate hydrogen gas with Na is X, which are soluble in NaOH is Y, which are neither soluble in NaOH nor liberate hydrogen gas with Na is Z then the value of (X-Y-Z) is
(A) 4
(B) 5
(C) 0
Date: 25-05-2022
Q: At 300 K, 0.5 g of Benzoic acid (solid) was subjected to combustion in a bomb calorimeter when the temperature of calorimeter system (including water) was found to rise by 0.55 degree Celsius. If the heat capacity of calorimeter including water is 23.85 kJ/K, then enthalpy of combustion (kJ/mol) is
(A) -3200.67
(B) -3201.24
(C) -3198.75
(D) None of these
Date: 24-06-2022
Q: What is the nature of an aq. Soln. whose pH is 7 at 40 degree Celsius?
(A) Acidic
(B) Basic
(C) Neutral
