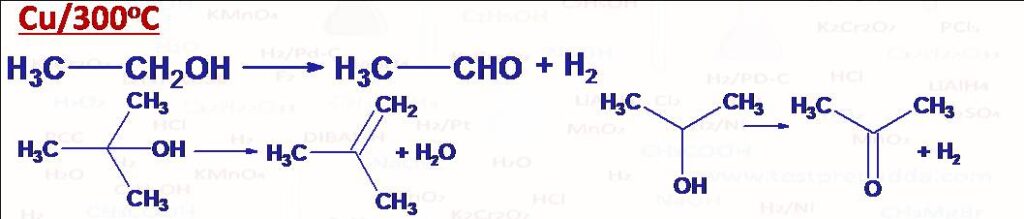
(1) Cu/300oC: (i) Dehydrogenation of primary alcohol into aldehyde. (ii) Dehydrogenation of secondary alcohol into ketone. (iii) Dehydration of tertiary alcohol into alkene.
(2) CYCLIC TRIMERISATION: When acetylene is passed through a copper tube heated to about 700oC, it undergoes cyclic trimerization involving three molecules to form benzene (poor yield). The same process can be carried out in good yield by catalysts Nickel cyanide Ni(CN)2 and triphenylphosphine (C6H5)3P (in combination).
(3) With a regulated supply of dioxygen and in the presence of catalyst Cu at 523K and 100 atm CH4 gets converted into CH3OH.
(4) Ullmann Reaction: Two molecules of aryliodide are coupled by heating with copper powder at around 250oC to form a biaryl. Two benzene rings are joined together. Aryl bromides and chlorides react only if electron withdrawing groups such as -CN or -NO2 are attached at ortho or para position.
