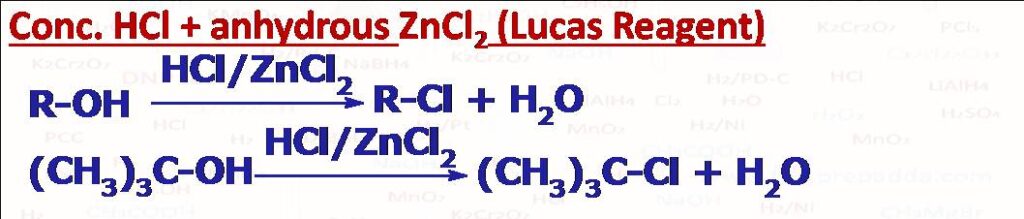
(1) Lucas Reagent Test (Conc.HCl + ZnCl2)
This is used to distinguish the three class of alcohols primary, secondary and tertiary. (1) If white turbidity (cloudiness) appears immediately it means alcohol is tertiary. (2) If white turbidity (cloudiness) appears within 5 minutes it means alcohol is secondary. (3) If cloudiness appears after a long time, this means alcohol is primary. Reaction of HCl with alcohol is a nucleophilic substitution reaction. Anhydrous ZnCl2 catalyzes the reaction by increasing the leaving group ability of -OH through its coordination with oxygen atom, we can also say that anhydrous ZnCl2 removes water (one product) from the reaction by absorbing it so reaction moves more in the forward direction. Please note that allyl (CH2=CH-CH2-OH) and benzyl (C6H5CH2-OH) alcohols also give this test even though these are primary.
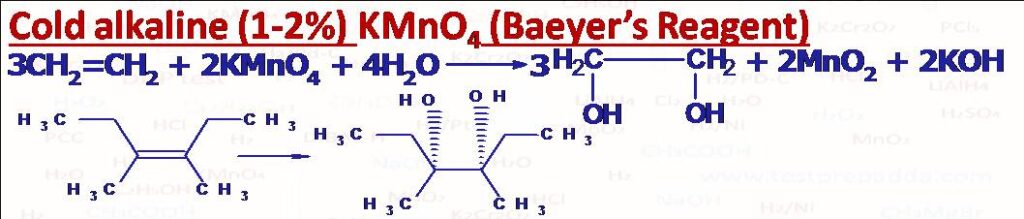
(2) Baeyer’s Reagent (Cold alkaline KMnO4) (1%)
This solution has purple (or lilac) colour and mainly used to test whether compound has double and triple bonds or not. After the reaction the colour disappears and brown precipitate of MnO2 forms.
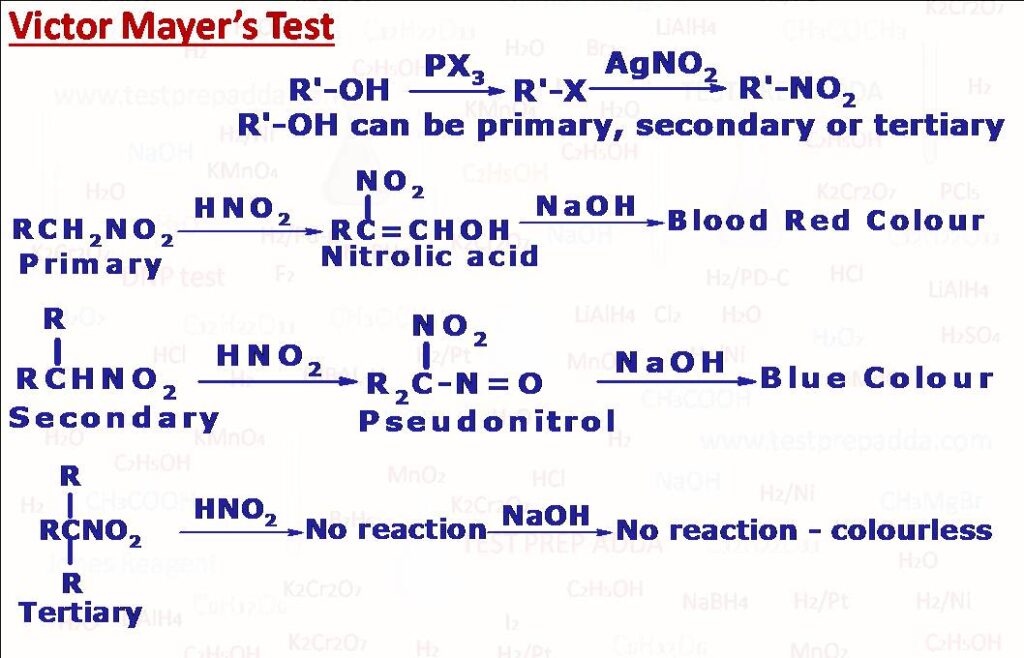
(3) Victor Mayer’s Test
This is used to distinguish the three class of alcohols primary(1 o) secondary (2 o and tertiary (3o) alcohols. The given alcohol is first converted into alkyl halide by reaction with PX3 or I2/red P then the formed alkyl halide is treated with AgNO2 to get nitroalkane. Nitroalkane when reacted with nitrous acid HNO2 and then made alkaline with excess of NaOH. If the resulting solution develops a blood red colour the alcohol is primary, if the resulting solution develops blue colour the alcohol is secondary and if no colour the alcohol is tertiary.
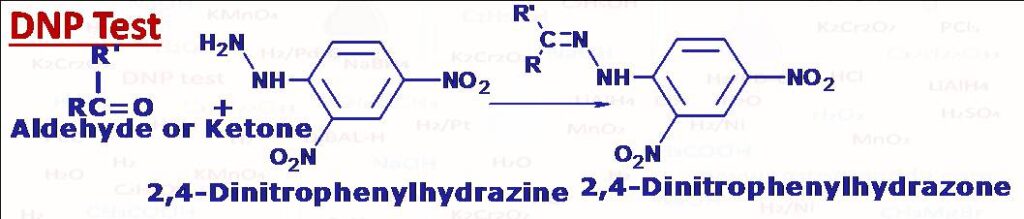
(4) DNP TEST (2,4-Dinitrophenylhydrazine-Brady’s reagent)
This test is used to check the presence of aldehydes or ketones in the compound. Aldehydes and ketones when heated with a solution of 2,4-Dinitrophenylhydrazine in ethanol containing small amount of H+ form corresponding 2,4-Dinitrophenylhydrazones. These are high melting-coloured solids (Orange, yellow or red). Aldehydes and ketones react with other ammonia derivatives in the same manner, like with primary amine they give imines with hydroxylamine they give oximes, with hydrazine give hydrazones.
(5) Neutral FeCl3 TEST (Test for phenol and enol)
This is used to a make distinction between a phenol (enol) and alcohol. The test is given by phenol not by alcohol. A violet colour develops after reaction due to formation of complex. Nearly all phenols give this test, however the colour may be violet, blue, green or red depending upon the structure of phenol. Enolic compounds also give this test.

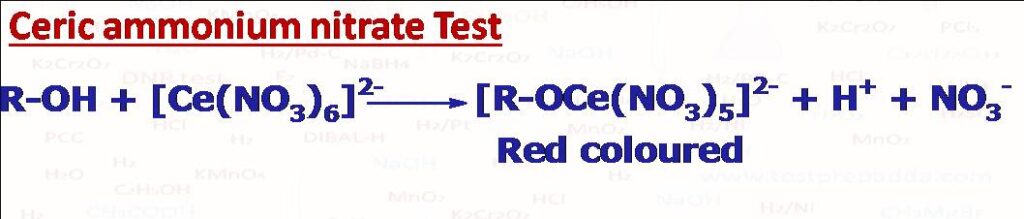
(6) CAN TEST (Ceric ammonium nitrate)
Alcohols give positive test with Ceric ammonium nitrate (NH4)2[Ce(NO3)6. The immediate formation of red or red-brown colour indicates positive test.
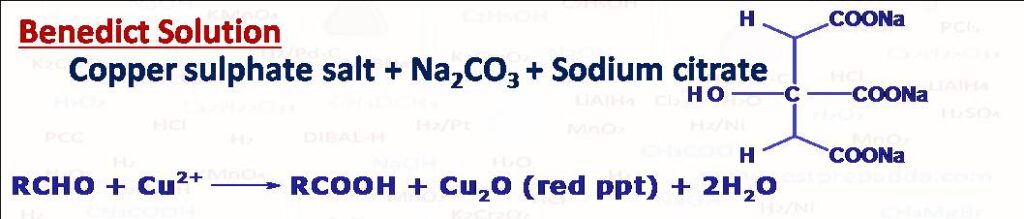
(7) Benedict Solution
The test is mainly used to distinguish between aldehydes and ketones. Test is given by aliphatic aldehydes and not by aromatic aldehydes. Red precipitate of cuprous oxide forms after the reaction. Benedict solution is alkaline solution of cupric ion complexed with citrate ion. The solution (deep blue) can be prepared by mixing copper sulphate, Na2CO3 and sodium citrate.
(8) Schiff Reagent Test
The test is mainly used to differentiate between aldehydes and ketones. This test is given by aldehydes. Ketones do not respond to this test.

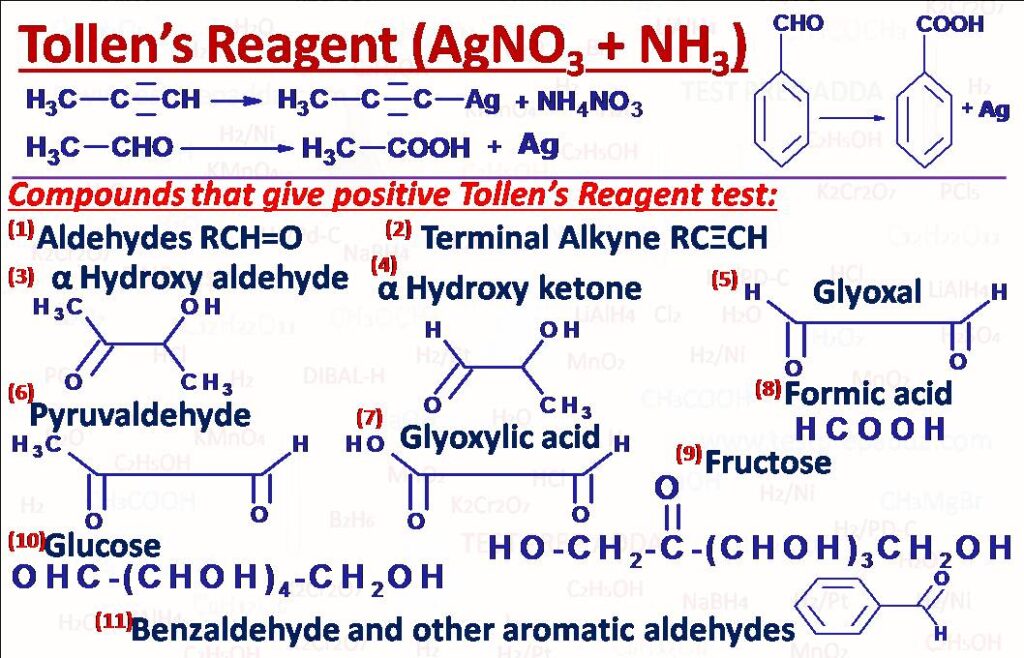
(9) Tollen’s Reagent Test
This test is used mainly to differentiate between aldehydes and ketones. Tollen’s reagent is an ammoniacal solution of silver nitrate in which silver ions are present in form as complex [Ag(NH3)2]+. This is considered to be mild oxidising agent so aldehydes get oxidised and ketones remain unaffected. Double and triple bonds also remain unaffected but terminal alkyne gives white ppt with Tollen’s reagent. During reaction silver ions get reduced to metallic silver and deposit on wall of test tube in the form of mirror, that is why this test is also known as SILVER MIRROR TEST. This test can be used for Aliphatic Aldehydes, Aromatic aldehydes, Alphahydroxyaldehydes, Alphahydroxyketones, Glyoxal, Glyoxylic acid, Formic acid, Succinaldehyde, Pyruvaldehyde, Glucose, Fructose etc. This Tollen\’s reagent is better oxidising agent than Fehling’s solution. Tollen’s reagent test is also given by terminal alkyne (white precipitate is formed).
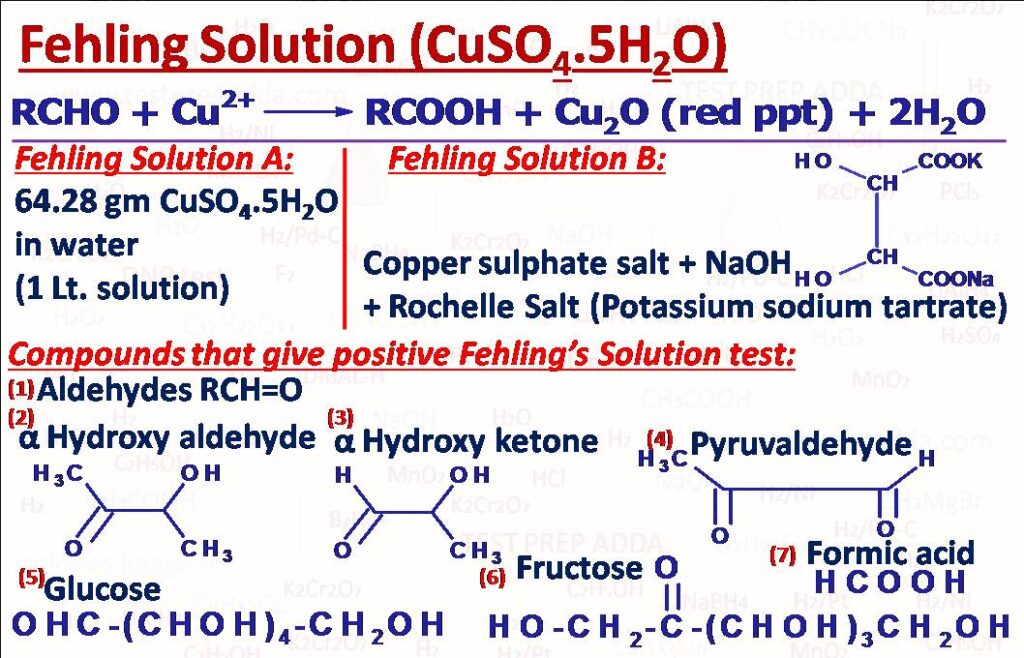
(10) Fehling Solution Test
This test is used mainly to differentiate between aldehydes and ketones. We can use Fehling solution A and Fehling solution B for the test. Fehling solution A is obtained by dissolving 64.28 gm CuSO4 in water and making the solution 1 litre. Fehling solution B is actually a complex copper tartrate formed by mixing CuSO4 salt in aqueous NaOH and Potassium sodium tartrate (Rochelle salt). The solution is deep blue in colour. Red precipitate forms after the reaction. Test is given by Aliphatic Aldehydes not aromatic aldehydes, Alphahydroxyaldehydes, Alphahydroxyketones, Formic acid, Succinaldehyde, Pyruvaldehyde, Glucose, Fructose etc. Double and triple bonds remain unaffected.
(11) Sodium bicarbonate test
It (NaHCO3) is used to test carboxylic acid (evolution of CO2) All compounds which are more acidic than H2O3 can be dissolved in H2CO3 and CO2 is evolved. Phenols are not soluble in NaHCO3, although polynitrophenols like 2,4-Dinitrophenol, 2,4,6-Trinitrophenol (also called picric acid) are soluble in NaHCO3.
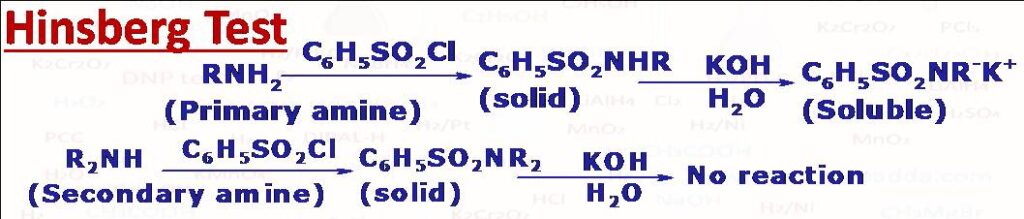
(12) Hinsberg Test
This test is used to differentiate between primary, secondary and tertiary amines. The given amine is shaken with benzenesulphonyl chloride in the presence of an aqueous KOH. Primary and scondary amines form substituted sulphonamides while tertiary amines do not. A primary amine forms N-alkylbenzenesulphonamide as a white solid which dissolves in aqueous KOH in the form of a salt (a clear solution), upon acidification an insoluble material separates. It forms salt with KOH since it has acidic H on the N atom. The secondary amine forms N,N-Dialkylbenzenesulphonamide as a white solid which does not dissolve in KOH. It does not react (dissolve) with KOH to form salt because it has no acidic H on nitrogen atom or elsewhere. The tertiary amine does not react with benzenesulphonylchloride since it has no hydrogen on N atom.
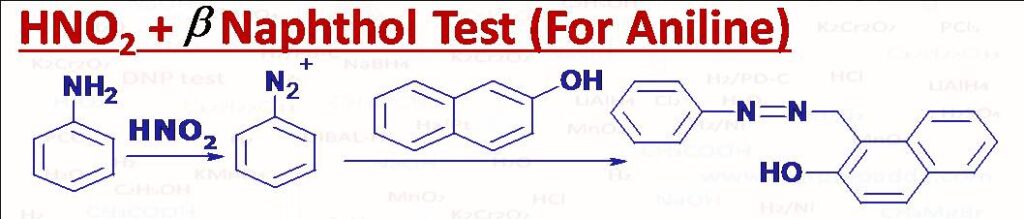
(13) HNO2 (or NaNO2 + HCl) and beta naphthol
It is used to test aniline (orange red dye is formed). Coupling Reaction.
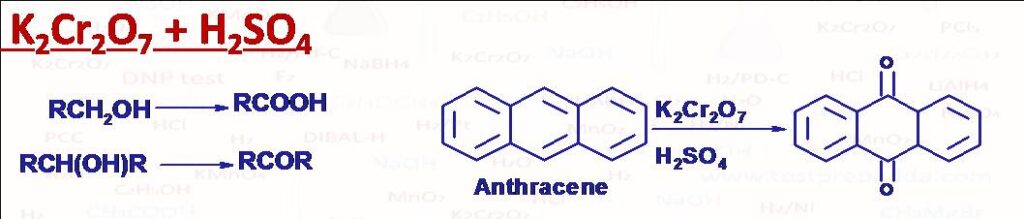
(14) Dichromate Test (K2Cr2O7 + H2SO4) (Orange Coloured Solution)
Primary alcohols are oxidised to carboxylic acid (Orange colour changes to green)while secondary alcohols are oxidised to ketones (Orange colour changes to green), Tertiary alcohols ate not oxidised directly under ordinary conditions as no alpha hydrogen present (no change in colour) but under suitable temperature it may undergo dehydration first to give = bond then double bond cleavage into mixture of ketones and carboxylic acids. Anthracene is converted into 9,10-Anthraquinone and Phenanthrene is converted into 9,10-Phenanthrenequinone.