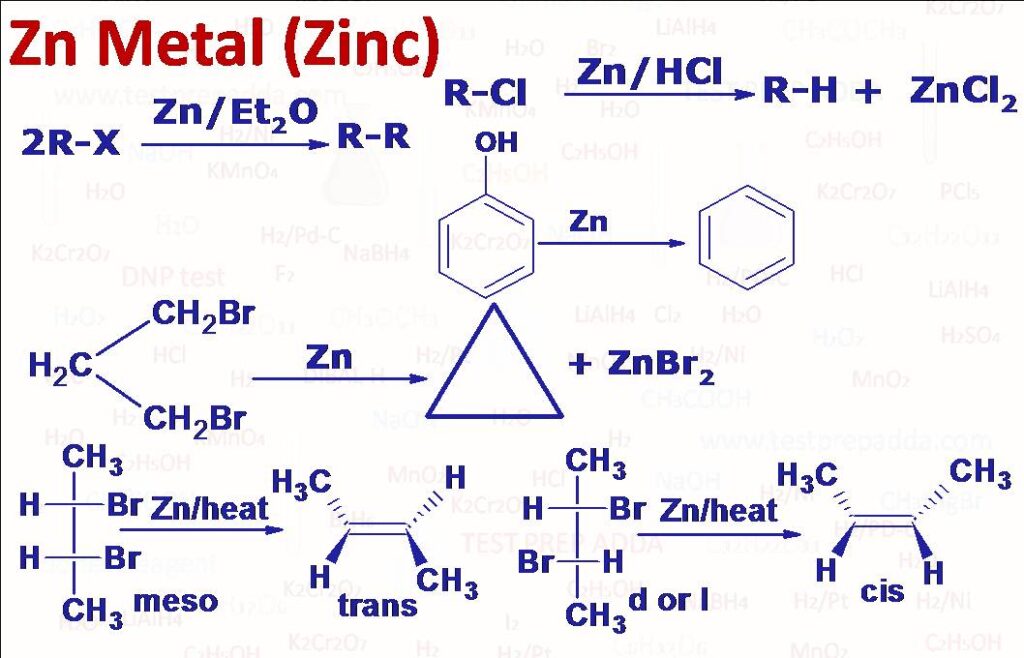
(1) FRANKLAND REDUCTION: When an alkylhalide (R-X) is heated with Zn in a solvent such as diethylether, a symmetrical alkane (R-R) is formed. The reaction is known as Frankland Reaction.
(2) Dehalogenation: On heating vicinal dihalides with Zn (Mg can also be used, NaI/C2H5OH can also be used) in solvent such as ethanol or acetic acid two halogen atoms get eliminated and alkene forms. The reaction follows E2 mechanism (one step with no intermediate) (beta elimination) with anti-elimination (Antiperiplanar). The reaction is stereospecific, meso-2,3-Dibromobutane on dehalogenation gives trand-But-2-ene and d or l 2,3-Dibromobutane on dehalogenation gives cis-But-2-ene. Vicinal dihalides are compounds which have two halogen atoms attached on adjacent carbon atoms. Geminal dihalides (two halogen atoms attached with same carbon) also give alkene by heating with Zn by first forming a carbene (alpha elimination). Dihalides in which the two halogen atoms are in the positions 1,3 to 1,6 give cyclic compounds. 1,4-Dibromobut-2-yne is debrominated to cumulene (CH2=C=C=CH2 (extended allene type of linkage). 2,3-Dibromopropylene can be debrominated to propadiene (allene) by Zn//C2H5OH.
(3) Zn/HCl: An alkyl halide (R-X) on treatment with either Zn and HCl or Zn and acetic acid or Zn-Cu/C C2H5OH gives corresponding alkane (R-H). This type of reduction is called dissolving metal reduction where an electron donor metal and proton donor acid are used.
(4) Phenol on distillation with Zn dust give benzene.
